- Review
- Open access
- Published:
A practical guide for the diagnosis of abdominal angiostrongyliasis caused by the nematode Angiostrongylus costaricensis
Parasites & Vectors volume 16, Article number: 155 (2023)
Abstract
Abdominal angiostrongyliasis (AA) is a severe parasitic infection caused by the nematode Angiostrongylus costaricensis. This disease is characterized by abdominal pain, a strong inflammatory eosinophilic response in the blood and tissues, and eventually intestinal perforation. Diagnosis of AA is challenging since there are no commercially available serological kits for A. costaricensis, and thus, histopathological analysis remains the gold standard. Herein we provide a decision flowchart for clinicians to improve the diagnosis of AA based on a patient’s clinical manifestations, laboratory findings, macroscopic observations of the gut lesions, as well as characteristic microscopic alterations in biopsies. A brief discussion of the available polymerase chain reaction and in-house serological methods is also presented. The aim of this mini-review is to improve the diagnosis of AA, which should lead to prompt detection of cases and better estimates of the epidemiology and geographical distribution of A. costaricensis.
Graphical Abstract

Background
Angiostrongylus costaricensis (family Angiostrongylidae) is a parasitic nematode of rodents that can be transmitted to humans by the ingestion of infected slugs or snails and is the agent responsible for abdominal angiostrongyliasis (AA), a severe intestinal disease of humans [1, 2]. Angiostrongylus costaricensis is closely related to the rat lungworm Angiostrongylus cantonensis which induces eosinophilic meningoencephalitis in humans [3]. Specific environmental conditions are necessary for the completion of A. costaricensis’ life cycle, such as warm temperatures and high humidity, which are favored by the abundant rainfall of tropical and subtropical South and North America [4]. This parasite has been reported in 24 countries of the Americas and the Caribbean, from the Southern United States to Northern Argentina [5], in which all the abiotic conditions for the parasite’s development are met. In these geographical locations, the nematode has been reported to cause infections in humans, or has been detected in its natural intermediate or definitive hosts.
The life cycle of A. costaricensis involves slugs or snails as intermediate hosts [2] and rodents as definitive hosts. There have also been a few reports of this parasite in domestic dogs, and wild animals, such as raccoons, non-human primates, and opossums [2, 6,7,8]. Third-stage larvae present in fibromuscular tissues or slime of snails or slugs [1] are ingested by definitive hosts, in which they follow a complex lymphatic-venous-arterial pathway until they reach the mesenteric or ileocolic artery [9]. In this final niche, the worms develop into male and female adults which copulate; the eggs are released into the gut mucosa where they concomitantly hatch into first-stage larvae [10]. The latter are released in the feces of the definitive host and can infect intermediate hosts via oral or transdermal pathways to finally develop into third-stage larvae in fibromuscular tissues or mucous gland ducts [11, 12]. Humans are considered accidental dead-end hosts since the hatching of eggs in their gut mucosa and the shedding of stage-one larvae in their feces are impaired by a strong eosinophilic response [13].
AA is mainly reported in school-age children and young adults [2], and is characterized by abdominal pain and blood/tissue eosinophilia, resulting from a severe inflammatory reaction, and eventually intestinal perforation [14,15,16]. These clinical manifestations are explained by the presence of adult worms in arteries and the strong eosinophilic response induced in situ. Extraintestinal complications are less frequently reported, but may include nodular hepatic lesions [17] or testicular necrosis [18]. In this guide, we propose a diagnostic flowchart to aid clinicians in the diagnosis of AA (Fig. 1), starting with the identification of clinical manifestations in patients, followed by the general as well as A. costaricensis-specific laboratory tests that need to be performed, and concluding with the main histopathological findings on biopsies.
Recommended decision flowchart for the diagnosis of abdominal angiostrongyliasis (AA). When typical clinical manifestations of AA are first observed in patients, several laboratory tests should be performed, including measurement of inflammatory blood markers and a hemogram showing cell counts and leukocyte percentages. If no eosinophilia is found, other pathologies are suspected. However, if the patient has eosinophilia, a parasitic infection is presumed to be present. A complete coprological analysis should then be performed to discard the possibility that the infection is due to other gastrointestinal parasites. An immunoagglutination assay or enzyme-linked immunosorbent assay for Angiostrongylus costaricensis is recommended if no parasitic agent is found during coprological analysis. If the latter assays are negative, a computed tomography scan is recommended to rule out possible gut malignancies. However, if the enzyme-linked immunosorbent assay or immunoagglutination tests are positive, a biopsy of the affected gut section should be analyzed to confirm infection by A. costaricensis. Key macroscopic and microscopic alterations of the gut tissue infected with this parasite are indicated. ESS Erythrocyte sedimentation speed, RCP reactive C-protein, FDP fibrinogen-derived products. This figure was created using BioRender.com
Diagnosis of AA
Diagnosis of AA is generally confirmed by the identification of A. costaricensis eggs, larvae or adult worms during histopathological analysis of the vermiform appendix and small and large bowel [19], although rare cases of testicular and liver disease have also been reported [20,21,22]. It is also possible to confirm the diagnosis in suspected cases by polymerase chain reaction (PCR) using DNA from formalin-fixed paraffin-embedded tissues (FFPE) [23]. Nucleic acid detection in serum has also been standardized for AA using primers targeting A. cantonensis sequences [24, 25]. Studies are underway to design additional assays using A. costaricensis sequences.
Histopathology: macroscopic and microscopic findings
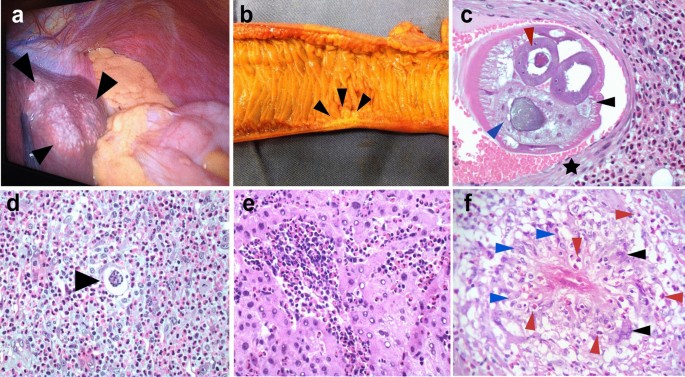
Macroscopically, an appendix infected with A. costaricensis is similar in appearance to that seen in routine cases of acute appendicitis, with red or black fibrinopurulent deposits in the serosa and a thickened wall (Fig. 2a, b) [13]. To increase the chances of finding parasitic structures during the microscopic analysis, when preparing the FFPE it is necessary to include the entire vermiform appendix and the mesoappendix [23].
Macroscopic and microscopic histopathological findings of AA. a Extraintestinal angiostrongyliasis in liver with multiple small nodules (black arrowheads) and yellowish material. b Small bowel showing a segmental Crohn disease-like lesion with wall thickening and hemorrhagic area (black arrowheads). c Transversal section of Angiostrongylus costaricensis adult female worm inside a branch of the mesenteric artery showing polymyarian musculature (black arrowhead), uterus with an egg inside (red arrowhead) and gut (blue arrowhead). Muscle cells of the artery are shown by a black star [hematoxylin–eosin staining (HE), ×200]. d Angiostrongylus costaricensis egg (black arrowhead) inside a small vessel surrounded by severe eosinophilic infiltration (HE, ×400). e Eosinophilic infiltration in the liver of a patient with AA (HE, ×400). f Granuloma with histiocytes (blue arrowheads), giant multinucleated cells (black arrowhead) and eosinophils (red arrowheads) (HE, ×400)
Two types of macroscopic lesions are observed in the bowel: intestinal infarction, and segmental and nodular lesions in the large bowel with thickened areas. There may be multiple segmental and nodular lesions, which may resemble Crohn disease [26] (Fig. 2b). It is important to perform adequate sampling of the infarcted lesions by randomly selecting areas of the intestinal wall and undertaking extensive sectioning of the mesentery. If the diagnosis is not confirmed from the first sampling, it is necessary to embed the entire surgical specimen in paraffin since parasitic structures may be absent from some sections. Moreover, blocks of the entire macroscopic lesion are required when sampling segmental or nodular lesions. In these cases, samples of the mesentery should be taken only from the hemorrhagic sites or thickened vessels [23]. It is crucial to carefully analyze the antimesenteric portion of the bowel, where eggs or larvae may be found in capillary lumens [13].
Routine hematoxylin and eosin staining is used for microscopic analysis of AA. The diagnosis of AA is confirmed when eggs, larvae or sections of adult worms are found in the lumens of capillaries, arterioles and large arteries [5, 23]. Adult worms are found mainly in submucosal, muscular, serosa or large mesenteric/mesoappendix arteries, where they may be associated with thrombosis and infarction (Fig. 2c) [27, 28]. In our experience, the majority of adult worms are found in the submucosal and mesenteric arteries.
A type 2 inflammatory response induced by A. costaricensis generates a strong eosinophilic infiltration [5], a key observation during the microscopic examination of biopsies (Fig. 2c–f). Eosinophilic infiltration around capillaries and arterioles of the submucosa and muscularis propria is associated with severe disease. Eosinophilic infiltration of arterial walls, which is termed eosinophilic arteritis, is a histopathological feature of AA (Fig. 2c) [26]. Additionally, granulomas are observed in the walls of large arteries [26] or engulfing capillaries and arterioles, together with the presence of eggs or larva in the lumen [5, 29]. Severe granulomatous reactions in the submucosa and muscularis propria of the large bowel leads to pseudotumor formation together with small vessel occlusion due to inflammation. Necrosis of the mucosa or intestinal wall and secondary ulceration may occur in severe disease [29].
AA is associated with characteristic clinical manifestations in patients, the observation of eosinophilic infiltration, eosinophilic arteritis and granulomas engulfing capillaries and arterioles, as well as key epidemiological features. Sometimes it is necessary to include the complete surgical specimen in FFPE blocks and analyze serial slides of the suspected infected areas to identify parasitic structures.
In-house serological methods
In-house serological assays like a latex agglutination test (Morera test) and an immunoglobulin G (IgG)–enzyme-linked immunosorbent assay are available in Costa Rica (from the Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud, Cartago) and Brazil (from the Instituto Adolfo Lutz, São Paulo), and both use whole somatic A. costaricensis antigens [30]. Crude antigens from eggs have been evaluated for serological assays [31] but are not used in routine diagnostic tests.
Difficulties in obtaining large numbers of A. costaricensis adult specimens and maintaining the parasite’s life cycle have prompted the use of whole crude antigens [32] and recombinant proteins (galectin) [33] from its congeneric species A. cantonensis for serological testing [32], which have proven successful. Since these assays use heterologous proteins of A. cantonensis, epidemiological factors as well as the patient’s clinical history and manifestations should be considered for the correct interpretation of the results and to discard infection with A. cantonensis. It is highly likely that future protocols will involve rapid tests that use homologous recombinant antigens [33, 34]. Besides improving the reproducibility of these assays, highly purified and well-characterized A. costaricensis antigens may prevent cross-reactivity with other nematode species, and specifically to the threadworm Strongyloides stercoralis [31, 35]. Increased specificity may also result from the detection of IgG1 antibodies, as suggested by Abrahams-Sandi et al. [31]. The reactivity of human IgG decreases with time in post-acute infections, but it may remain detectable for several months [36].
PCR methods
Three DNA-based methods have been designed for confirming the diagnosis of AA. The first method was a conventional end-point PCR which used a 232-base pair fragment of a 66-kDa muscle protein of female A. cantonensis (Ac-fmp-1) as a target. This reaction detected the Ac-fmp-1 homologue in A. costaricensis [24] in the sera of two out of three patients with AA. Moreover, the PCR did not cross-amplify DNA of other gastrointestinal nematodes such as Strongyloides ratti, Ancylostoma caninum, Ascaris suum, and Toxocara canis [24].
The second conventional end-point PCR method used FFPE samples from patients with confirmed AA to detect the same Ac-fmp-1 homologous DNA fragment of A. costaricensis [23]. This method detected 55% (11/20) of cases confirmed by histopathology, especially in sections containing parasitic structures or granulomas [23]. Overall, this PCR showed intermediate sensitivity and high specificity, since the reactions were negative for FFPE samples of negative controls and FFPE samples with Ascaris lumbricoides, Enterobius vermicularis, Strongyloides stercoralis and Schistosoma mansoni [23].
The third molecular assay was a real-time PCR that also amplified a DNA fragment of the Ac-fmp-1 of A. costaricensis in sera of patients with presumptive AA [25]. This real-time PCR detected the parasite’s DNA in two out of 28 sera matched to patients with AA. In addition, the two samples positive in the real-time PCR were negative according to an indirect enzyme-linked immunosorbent assay. Therefore, the method confirmed the presence of the nematode’s DNA in sera of patients with suspected AA and complemented the results of serological techniques, suggesting that the assay might be useful during the acute phase of the infection [25].
Conclusions
An interdisciplinary approach is needed to solve the current challenges in the diagnosis of AA. This approach includes education as well as regularly updating healthcare professionals and pathologists about the clinical characteristics of this parasitosis, which in turn should promote awareness of potential AA cases in geographic regions where the infection has not been reported before. Finally, the histopathological criteria summarized here should be adequate for the diagnosis of AA, and may help us better understand the epidemiology and distribution of this parasite. However, further studies are required to develop a sensitive and specific molecular diagnostic tool for AA to improve the quality of the clinical approach for patients with this disease by reducing the amount of time lost prior to diagnosis and avoiding the risks that are associated with biopsy.
Availability of data and materials
Not applicable.
Abbreviations
- AA:
-
Abdominal angiostrongyliasis
- FFPE:
-
Formalin-fixed paraffin embedded
References
Bonetti VC, Graeff-Teixeira C. Angiostrongylus costaricensis and the intermediate hosts: observations on elimination of L3 in the mucus and inoculation of L1 through the tegument of mollucs. Rev Soc Bras Med Trop. 1998;31:289–94.
Solano-Barquero A, Mora J, Graeff-Teixeira C, Rojas A. Angiostrongylus costaricensis. Trends Parasitol. 2021;37:1111–1112.
Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostrongyliasis. Lancet Infect Dis. 2008;8:621–30.
Morera P, Amador JA. Prevalencia de la angiostrongilosis abdominal y la distribución estacional de la precipitación. Rev Cost Sal Pub. 1998;7:13.
Rojas A, Maldonado-Junior A, Mora J, Morassutti A, Rodriguez R, Solano-Barquero A, et al. Abdominal angiostrongyliasis in the Americas: fifty years since the discovery of a new metastrongylid species, Angiostrongylus costaricensis. Parasit Vectors. 2021;14:374.
Miller CL, Kinsella JM, Garner MM, Evans S, Gullett PA, Schmidt RE. Endemic infections of Parastrongylus (=Angiostrongylus) costaricensis in two species of nonhuman primates, raccoons, and an opossum from Miami. Florida J Parasitol. 2006;92:406–8.
Alfaro-Alarcon A, Veneziano V, Galiero G, Cerrone A, Gutierrez N, Chinchilla A, et al. First report of a naturally patent infection of Angiostrongylus costaricensis in a dog. Vet Parasitol. 2015;212:431–4.
Rodriguez R, Agostini AA, Porto SM, Olivaes AJ, Branco SL, Genro JP, et al. Dogs may be a reservoir host for Angiostrongylus costaricensis. Rev Inst Med Trop Sao Paulo. 2002;44:55–6.
Mota EM, Lenzi HL. Angiostrongylus costaricensis: complete redescription of the migratory pathways based on experimental Sigmodon hispidus infection. Mem Inst Oswaldo Cruz. 2005;100:407–20.
Arroyo R, Morera P. Viability of the first stage larvae of Angiostrongylus costaricensis in rat feces. J Parasitol. 1978;64:146.
Montresor LC, Vidigal TH, Mendonca CL, Fernandes AA, de Souza KN, Carvalho OS, et al. Angiostrongylus costaricensis (Nematoda: Protostrongylidae): migration route in experimental infection of Omalonyx sp. (Gastropoda: Succineidae). Parasitol Res. 2008;103:1339–46.
Mendonca CL, Carvalho OS, Mota EM, Lenzi HL. Development of Angiostrongylus costaricensis Morera and Cespedes 1971 (Nematoda: Angiostrongylidae) larvae in the intermediate host Sarasinula marginata (Semper 1885) (Mollusca: Soleolifera). Parasitol Res. 2008;102:861–5.
Morera P, Cespedes R. Angiostrongylus costaricensis n. sp. (Nematoda: Metastrongyloidea), a new lungworm occurring in man in Costa Rica. Rev Biol Trop. 1970;18:173–85.
Vargas M, Campos E, Mata C. Parasitología RNdLd: evaluación de test de Morera según resultados del Centro Nacional de Referencia de Parasitología—Inciensa. Costa Rica enero 2012—abril 2020. Parasitologia CNdRd eds. Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud 2020.
Mesén-Ramírez P, Calvo N. Diagnóstico de la angiostrongilosis abdominal en Costa Rica, CNRP, 2010. Parasitología CNdRe eds. Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud 2010.
Bolaños F, Jurado LF, Luna-Tavera RL, Jiménez JM. Abdominal angiostrongyliasis, report of two cases and analysis of published reports from Colombia. Biomedica. 2020;40:233–42.
Morera P, Perez F, Mora F, Castro L. Visceral larva migrans-like syndrome caused by Angiostrongylus costaricensis. Am J Trop Med Hyg. 1982;31:67–70.
Sanchez-Sierra LE, Martinez-Quiroz RA, Antunez HS, Cabrera-Interiano H, Barrientos-Melara FJ. Right testicular artery occlusion and acute appendicitis by Angiostrongylus costaricensis. Case Rep Surg. 2019;2019:5670802.
Graeff-Teixeira C, Camillo-Coura L, Lenzi H. Clinical and epidemiological aspects of abdominal angiostrongyliasis in southern Brazil. Rev Inst Med Trop Sao Paulo. 1991;33:373–8.
Ruiz PJ, Morera P. Spermatic artery obstruction caused by Angiostrongylus costaricensis Morera and Cespedes, 1971. Am J Trop Med Hyg. 1983;32:1458–9.
Loria-Cortes R, Lobo-Sanahuja JF. Clinical abdominal angiostrongylosis. A study of 116 children with intestinal eosinophilic granuloma caused by Angiostrongylus costaricensis. Am J Trop Med Hyg. 1980;29:538–44.
Rodriguez R, Dequi RM, Peruzzo L, Mesquita PM, Garcia E, Fornari F. Abdominal angiostrongyliasis: report of two cases with different clinical presentations. Rev Inst Med Trop Sao Paulo. 2008;50:339–41.
Rodriguez R, da Silva AC, Muller CA, Alves SL, Graeff-Teixeira C, Fornari F. PCR for the diagnosis of abdominal angiostrongyliasis in formalin-fixed paraffin-embedded human tissue. PLoS ONE. 2014;9:e93658.
Da Silva AC, Graeff-Teixeira C, Zaha A. Diagnosis of abdominal angiostrongyliasis by PCR from sera of patients. Rev Inst Med Trop Sao Paulo. 2003;45:292–7.
Amaral FUI, Zorzi NR, Soveral LF, Frandoloso R, Rodriguez R, Graeff-Teixeira C, et al. Molecular diagnosis of abdominal angiostrongyliasis by PCR using serum samples. Parasitol Res. 2022. https://doi.org/10.1007/s00436-022-07763-6.
Agostini AA, Marcolan AM, Lisot JMC, Lisot JUT. Estudo anátomo-patológico de quatro casos observados no Rio Grande do Sul, Brasil. Mem Inst Oswaldo Cruz. 1984;79:443–5.
Sierra E, Morera P. Angiostrongilosis abdominal. Primer caso encontrado en Honduras. Acta Med Costarr. 1972;15:95–9.
Iabuki K, Montenegro MR. Appendicitis caused by Angiostrongylus costaricensis. Presentation of a case. Rev Inst Med Trop Sao Paulo. 1979;21:33–6.
Rodriguez R, Porto SM, Dos Santos FR, Marcolan AM, da Silva AC, Graeff-Teixeira C, et al. Outcomes in mice with abdominal angiostrongyliasis treated with enoxaparin. Parasitol Res. 2011;109:787–92.
Geiger SM, Laitano AC, Sievers-Tostes C, Agostini AA, Schulz-Key H, Graeff-Teixeira C. Detection of the acute phase of abdominal angiostrongyliasis with a parasite-specific IgG enzyme linked immunosorbent assay. Mem Inst Oswaldo Cruz. 2001;96:515–8.
Abrahams-Sandi E, Mesén-Ramírez P, Suarez-Chacón D, Fernández-Quesada K. An indirect immunofluorescence antibody test employing whole eggs as the antigen for the diagnosis of abdominal angiostrongyliasis. Mem Inst Oswaldo Cruz. 2011;106:390–3.
Ben R, Rodrigues R, Agostini AA, Graeff-Teixeira C. Use of heterologous antigens for the immunodiagnosis of abdominal angiostrongyliasis by an enzyme-linked immunosorbent assay. Mem Inst Oswaldo Cruz. 2010;105:914–7.
Graeff-Teixeira C, Pascoal VF, Rodriguez R, Morassutti AL, Intapan PM, Maleewong W. Abdominal angiostrongyliasis can be diagnosed with a immunochromatographic rapid test with recombinant galactin from Angiostrongylus cantonensis. Mem Inst Oswaldo Cruz. 2020;115:e200201.
Morassutti AL, Perelygin A, Levert K, Lin SC, Lee YM, da Silva AJ, et al. Expression of recombinant antigenic proteins from Angiostrongylus cantonensis: a brief report. Hawaii J Med Public Health. 2013;72:58–62.
Kramer MH, Greer GJ, Quinonez JF, Padilla NR, Hernandez B, Arana BA, et al. First reported outbreak of abdominal angiostrongyliasis. Clin Infect Dis. 1998;26:365–72.
Palominos PE, Gasnier R, Rodriguez R, Agostini AA, Graeff-Teixeira C. Individual serological follow-up of patients with suspected or confirmed abdominal angiostrongyliasis. Mem Inst Oswaldo Cruz. 2008;103:93–7.
Acknowledgements
The authors would like to thank Carlos Mata (Parasitology Reference Laboratory, Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud, Costa Rica) and Leyva Cecilia Viera de Melo (Instituto Adolfo Lutz, São Paulo).
Funding
This work was supported by the Vicerrectoria de Investigacion, Universidad de Costa Rica (project C2064).
Author information
Authors and Affiliations
Contributions
AR, RR, CG-T conceived the manuscript; AR prepared the flowchart diagram; RR and AR prepared the histopathology pictures; RR, AR, AS-B, JM, CG-T wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rodriguez, R., Mora, J., Solano-Barquero, A. et al. A practical guide for the diagnosis of abdominal angiostrongyliasis caused by the nematode Angiostrongylus costaricensis. Parasites Vectors 16, 155 (2023). https://doi.org/10.1186/s13071-023-05757-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05757-6