- Brief Report
- Open access
- Published:
Prevalence of non-falciparum malaria infections among asymptomatic individuals in four regions of Mainland Tanzania
Parasites & Vectors volume 17, Article number: 153 (2024)
Abstract
Background
Recent studies point to the need to incorporate the detection of non-falciparum species into malaria surveillance activities in sub-Saharan Africa, where 95% of the world’s malaria cases occur. Although malaria caused by infection with Plasmodium falciparum is typically more severe than malaria caused by the non-falciparum Plasmodium species P. malariae, P. ovale spp. and P. vivax, the latter may be more challenging to diagnose, treat, control and ultimately eliminate. The prevalence of non-falciparum species throughout sub-Saharan Africa is poorly defined. Tanzania has geographical heterogeneity in transmission levels but an overall high malaria burden.
Methods
To estimate the prevalence of malaria species in Mainland Tanzania, we randomly selected 1428 samples from 6005 asymptomatic isolates collected in previous cross-sectional community surveys across four regions and analyzed these by quantitative PCR to detect and identify the Plasmodium species.
Results
Plasmodium falciparum was the most prevalent species in all samples, with P. malariae and P. ovale spp. detected at a lower prevalence (< 5%) in all four regions; P. vivax was not detected in any sample.
Conclusions
The results of this study indicate that malaria elimination efforts in Tanzania will need to account for and enhance surveillance of these non-falciparum species.
Graphical Abstract
Tanzania has one of the highest malaria burdens in the world, accounting for 3.1% of global malaria cases and 4.1% of global malaria deaths in 2021 [1]. While most malaria cases in Tanzania and elsewhere in sub-Saharan Africa are caused by Plasmodium falciparum, four other Plasmodium species (P. vivax, P. malariae, P. ovale curtisi and P. ovale wallikeri) are present to varying degrees. Current data also suggest that the prevalence of these species is higher than previously believed and that they may become even more prevalent as P. falciparum is controlled and ultimately eliminated [2,3,4,5,6], in line with the WHO goal of a 90% reduction in global malaria burden from 2015 levels by 2030 [7]. Non-falciparum malaria (i.e. malaria infection due to Plasmodium species other than P. falciparum) may require different control measures due to major differences in biology, including different Anopheles vectors with different seasonal peaks [8], relapse and/or chronic infections [9, 10], lower parasitemia [11] and higher rates of asymptomatic infection [8].
Previous work in Mainland Tanzania has characterized the prevalence of non-falciparum malaria in school children (age: 5–16 years) [6] and non-falciparum positivity rates among symptomatic patients [12]. In the school children, the prevalence of P. ovale spp. malaria infections (24%) was similar to that of P. falciparum (22%) [6], while in symptomatic patients, P. falciparum malaria infections were much more abundant than non-falciparum malaria, although P. ovale spp. positivity rates surpassed 5% in seven of 10 regions [12]. In both studies, P. malariae was less common than either P. falciparum or P. ovale spp., and P. vivax was rare [6, 12]. However, a full characterization of non-falciparum prevalence in Mainland Tanzania requires accounting for asymptomatic individuals of all ages, who may act as potential reservoirs. In the study reported here, we characterized the prevalence of all malaria species among asymptomatic individuals across all ages in three regions with moderate and high malaria transmission intensity, and in children aged < 5 years in one region with high transmission.
The study protocol was approved by the Tanzanian Medical Research Coordinating Committee (MRCC) of the National Institute for Medical Research (NIMR) and involved approved standard procedures for informed consent and sample deidentification. Additional details are described elsewhere [13]. Deidentified samples were considered non-human subjects’ research at the University of North Carolina and Brown University.
A random subset of 1428 dried blood spot (DBS) samples were drawn from a total of 5860 asymptomatic samples, including 694 samples from a total of 2647 collected from all age groups [12] during cross-sectional community surveys in the Kigoma (n = 252/878, high transmission area), Ruvuma (n = 186/741, high transmission area) and Tanga (n = 256/1028, moderate transmission area) regions during the Molecular Surveillance of Malaria in Tanzania (MSMT) project in 2021 [13]. The random subset was representative of the regional sample distribution (χ2 = 2.43, df = 2, P = 0.3; Additional file 1: Table S1), but not representative of the age group distribution (χ2 = 10.46, df = 2, P = 0.005; Additional file 1: Table S2), or the sex distribution (χ2 = 43.65, df = 1, P < 0.001; Additional file 1: Table S3). An additional 734 samples were drawn from 3213 samples collected from children aged < 5 years during cross-sectional household surveys for the Group Antenatal Care project (GANC) [14, 15] in Geita in 2021. In all regions, study participants were administered a malaria rapid diagnostic test (mRDT) in their communities. Participants with a positive test result were treated with artemether-lumefantrine and adjunct medications based on the presence of concurrent illnesses.
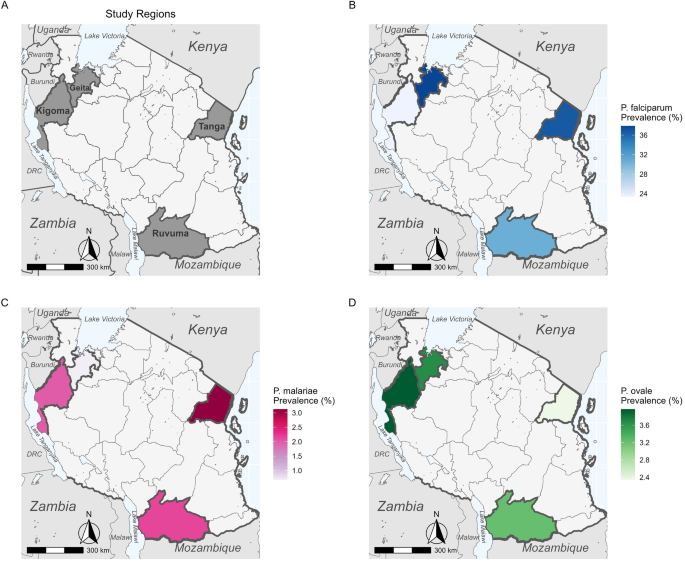
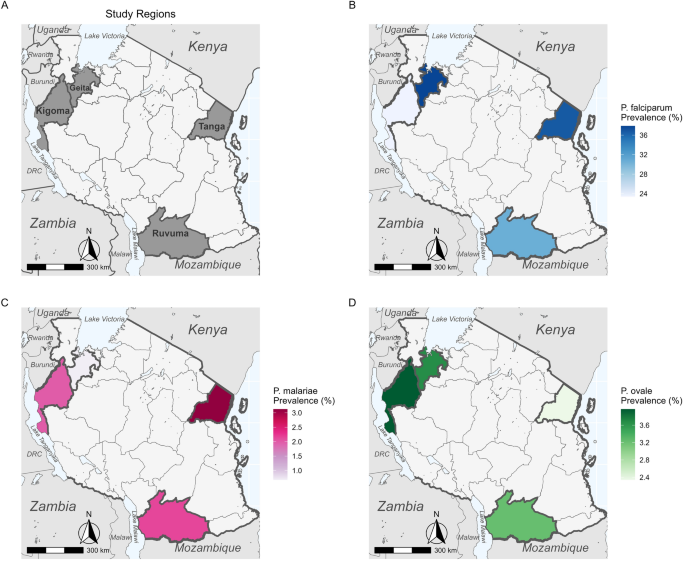
The molecular analyses used to detect Plasmodium spp. in each sample are described in detail elsewhere [12]. Briefly, we performed a separate quantitative PCR (qPCR) assay targeting the 18S ribosomal RNA gene (18S rRNA) for each species, which allows for both the detection of each species as well as a semi-quantitative estimate of parasitemia. For each region, we calculated prevalence for each species, including both single-species and mixed-species infections. Regional-level maps of prevalence for each species were created using the R package sf (version 1.0.9; R Foundation for Statistical Computing, Vienna, Austria) based on shape files available from GADM.org and naturalearthdata.com accessed via the R package rnaturalearth (version 0.3.2) [16]. Variation in species-specific prevalence by region and age group was assessed for significance with generalized linear models (GLMs) or analysis of variance (ANOVA), as appropriate, in R.
Excluding the Geita participants who were all aged < 5 years and whose ages were not recorded, the median age of the remaining 694 participants was 20 (interquartile range [IQR] 8–47) years (range 6 months to 87 years). Including the Geita participants, children (≤ 16 years old) constituted 74.2% of participants (n = 1060), while adults (> 16 years old) constituted the remaining 25.8% (n = 368). Young children (< 5 years old) comprised 77.9% (n = 826) of the pediatric participants, while the remaining 22.1% (n = 234) were school-aged children (5–16 years old). Sex identifications were available for 694 participants and were female-skewed, with 505 female (72.8%) and 189 male participants (27.2%). Of the sampled individuals, 21.1% (n = 301) were RDT-positive (Additional file 1: Table S4) using a standard HRP2/Pan RDT (First Response® [Premier Medical Corp., Ltd, Valsad, Gujarat, India]; SD Bioline™ [Abbott Diagnostics Ltd. Korea, Seoul, Republic of Korea]; or Care Start™ [Access Bio, Inc., Somerset, NJ, USA]).
Among all 1428 samples analyzed, P. falciparum was detected in 34.2% (n = 488, 95% confidence interval [CI] 31.7–36.7%), P. malariae in 1.5% (n = 22, 95% CI 0.99–2.4%) and P. ovale spp. in 3.4% (n = 49, 95% CI 2.6–4.5%); P. vivax was not detected in any sample. Plasmodium malariae infections were nearly evenly split between single-species infections (45.5%, n = 10/22) and mixed-species infections with P. falciparum (40.9%, n = 9/22), with the remaining three infections (13.6%) being triple infections with P. falciparum and P. ovale spp. (Table 1). In contrast, most P. ovale spp. infections were mixed with P. falciparum (65.3%, n = 32/49), with single-species infections being less common (28.6%, n = 14/49) and triple infections comprising the remainder (Table 1). The highest median parasitemia was recorded for Plasmodium malariae at 164,080 parasites/µl blood (IQR 9942–1333,100 p/µl), followed by P. falciparum at 55,200 (IQR 2910–775,000) and P. ovale spp. at 11,868 (IQR 1271–70,840) p/µl. However, there was no significant difference in parasitemia by species (F(2,556) = 0.085, P = 0.9).
All three Plasmodium species recorded in the samples (P. vivax was not detected in any sample) were detected in each region (Fig. 1). The highest P. falciparum prevalence was found in Geita and Tanga (37.8% [n = 278/734] and 36.7%, [94/256], respectively; Additional file 1: Table S5). Plasmodium malariae was relatively rare in all four regions, with the highest prevalence recorded in Tanga (3.1%, n = 8/256) and the lowest in Geita (0.7%, n = 5/734; Additional file 1: Table S5). Plasmodium ovale spp. prevalence was slightly higher than that of P. malariae (3.2–4.0%, n = 6/186–10/252) in all regions except Tanga (2.3%, n = 6/256; Additional file 1: Table S5). There was significant variation (by ANOVA) in prevalence between regions for P. falciparum (F(3,1424) = 6.47, P < 0.001) and P. malariae (F(3,1424) = 2.87, P = 0.0351), but not for P. ovale spp. (F(3,1424) = 0.43, P = 0.732).
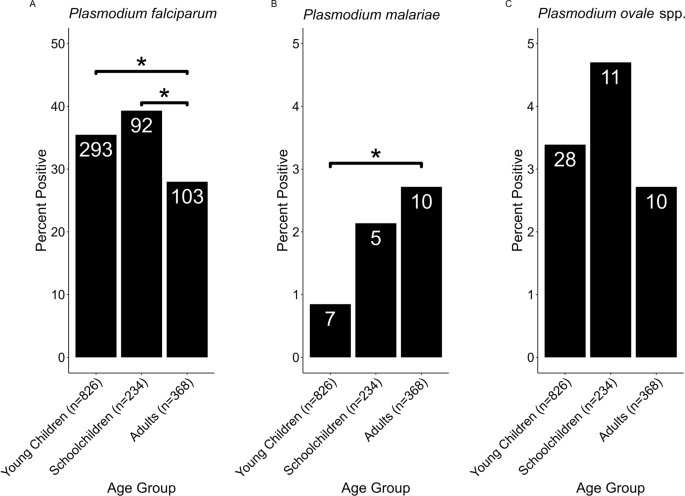
While age was a significant (GLM: t(1427) = 23.7, P < 0.001) determinant of P. falciparum infection, there was no significant effect of age for infection with either P. malariae or P. ovale spp. Age group was found to be a significant determinant of infection likelihood for both P. falciparum (F(2,1418) = 4.92, P = 0.007) and P. malariae (F(3,1418) = 3.28, P = 0.0381), but not for P. ovale spp. (F(3,1418) = 0.857, P = 0.425) (Fig. 2). While children were significantly (Tukey HSD test: P < 0.05) more likely than adults to have P. falciparum infection, adults were more likely to have P. malariae infection (Tukey HSD test: P < 0.05; Fig. 2). There was no significant interaction between age group and region for either P. falciparum or P. malariae infection, but the interaction was nearly significant (P = 0.055) for P. ovale spp. infection
Tukey HSD analysis of malaria species prevalence by age group. A total of 826, 234 and 368 samples were positive for malaria infection in the age groups ‘young children’ (< 5 years), ‘school children’ (5–16 years) and ‘adults’ (> 16 years), respectively. The total number of samples per group for each species is shown on the X-axis under each bar, and the number of positive samples for each group is shown in each bar. a, b, c Prevalence by age group of P. falciparum (a) P. malariae (b) and P. ovale spp. (c). All comparisons marked with an asterisk were significant at the P < 0.05 level; all other comparisons were statistically non-significant
This study builds on previous research with school children and patients with a clinical diagnosis of malaria to describe the prevalence of different malaria species within four regions of Mainland Tanzania. Although P. falciparum is the most prevalent species in Mainland Tanzania, we found that the prevalence of both P. malariae and P. ovale spp. surpassed 3% in at least one region, and could increase as P. falciparum is locally eliminated, as has been seen with non-falciparum species in other contexts [1, 4]. In contrast to a 2017 study involving school children, which found that the prevalence of P. ovale spp. was similar to that of P. falciparum [6], we found P. ovale spp. prevalence to be much lower than that of P. falciparum. In addition, the 2017 survey of school children [6] mostly identified P. ovale spp. as single-species infections and P. malariae as mixed infections with P. falciparum. In contrast, most of our samples with P. ovale spp. infection were mixed with P. falciparum, and we found similar proportions of single-species and mixed-species P. malariae infections. However, our sample sizes are small and may not necessarily be representative of the full picture, particularly in Geita where samples were only collected from children aged < 5 years. Also, our study included only four regions, of which only one (Tanga) overlaps with those included in the previous study, although we did include a wider age range.
In Tanga, we may have found lower P. ovale spp. prevalence than that reported in the previous study due to the inclusion of adults in our study, who are less likely to test positive for this species, whereas schoolchildren are a major asymptomatic infectious reservoir [17,18,19]; however, we did not replicate a significant difference in this study. In addition, the disruptions to malaria control caused by the COVID-19 pandemic, particularly in 2020–2021 [20], could have caused an increase in P. falciparum prevalence, which might account for the decreased prevalence of P. ovale if there is competition between the two species. Indeed, there was no difference in malaria prevalence in the villages of Magoda, Mamboleo and Mpapayu between 2019 (24.9%) and 2021 (24.5%; unpublished data). However, the prevalence dropped to 6.4% in 2022 following a return to the implementation of normal control activities (unpublished data), so further longitudinal studies may clarify the impact of resumed intense P. falciparum control. However, the malaria prevalence in Tanga dropped from 34.8% to 26.2% between 2020 and 2021 (unpublished data), so P. falciparum control in this region was likely effective during the course of this study, meaning that other factors, such as the inclusion of adults or rainfall levels, were likely a larger determinant of the lower P. ovale spp. prevalence in our study.
We did not find school children to be significantly more likely than adults or young children to test positive for either P. malariae or P. ovale spp. [12], but this trend, although not significant, was recorded for P. ovale spp. prevalence in this study. Therefore, the lack of significance in these species is likely an artifact of small sample sizes (Table 1; Fig. 2, Additional file 2: Fig. S1). Our finding that P. malariae was significantly more prevalent among adults likely reflects the presence of chronic infections that are more likely to be found in adults than in children due to the inherently larger number of infection opportunities [10].
Although P. falciparum remains the most prevalent species in these four regions, P. malariae and P. ovale spp. are present in all four regions, whereas P. vivax was not detected. Achieving malaria elimination in Tanzania will require ongoing surveillance of and targeted interventions for these species. While standard treatments successfully clear P. falciparum, the 3.4% of patients in this study with P. ovale spp. malaria infection may relapse. This study serves as a complement to previous studies focusing on school children and symptomatic patients and provides a full picture of the non-falciparum malaria landscape for communities in Mainland Tanzania. Ongoing analysis of samples collected in 2022 and 2023 will allow us to detect temporal trends in prevalence, and a forthcoming genomic analysis of P. malariae and P. ovale spp. isolates from Tanzania will inform our understanding of population structure and diversity in these species.
Availability of data and materials
Data are available upon reasonable request to the corresponding author.
References
WHO. World malaria report 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022. Accessed 24 Mar 2023
Akala HM, Watson OJ, Mitei KK, Juma DW, Verity R, Ingasia LA, et al. Plasmodium interspecies interactions during a period of increasing prevalence of Plasmodium ovale in symptomatic individuals seeking treatment: an observational study. Lancet Microbe. 2021;2:e141–50.
Betson M, Clifford S, Stanton M, Kabatereine NB, Stothard JR. Emergence of nonfalciparum Plasmodium infection despite regular artemisinin combination therapy in an 18-month longitudinal study of Ugandan children and their mothers. J Infect Dis. 2018;217:1099–109.
Yman V, Wandell G, Mutemi DD, Miglar A, Asghar M, Hammar U, et al. Persistent transmission of Plasmodium malariae and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in eastern Tanzania. PLOS Negl Trop Dis. 2019;13:e0007414.
Nguiffo-Nguete D, Nongley Nkemngo F, Ndo C, Agbor J-P, Boussougou-Sambe ST, Salako Djogbénou L, et al. Plasmodium malariae contributes to high levels of malaria transmission in a forest–savannah transition area in Cameroon. Parasit Vectors. 2023;16:1–10.
Sendor R, Mitchell CL, Chacky F, Mohamed A, Mhamilawa LE, Molteni F, et al. Similar prevalence of Plasmodium falciparum and non-P falciparum malaria infections among Schoolchildren, Tanzania. Emerg Infect Dis. 2023;29:1143–53.
WHO. Global technical strategy for malaria 2016–2030. 2015. https://www.who.int/docs/default-source/documents/global-technical-strategy-for-malaria-2016-2030.pdf. Accessed 16 Jul 2021
Tarimo BB, Nyasembe VO, Ngasala B, Basham C, Rutagi IJ, Muller M, et al. Seasonality and transmissibility of Plasmodium ovale in Bagamoyo district, Tanzania. Parasit Vectors. 2022;15:56.
Collins WE, Jeffery GM. Plasmodium ovale: parasite and disease. Clin Microbiol Rev. 2005;18:570–81.
Oriero EC, Amenga-Etego L, Ishengoma DS, Amambua-Ngwa A. Plasmodium malariae, current knowledge and future research opportunities on a neglected malaria parasite species. Crit Rev Microbiol. 2021;0:1–13.
Roucher C, Rogier C, Sokhna C, Tall A, Trape JF. A 20-year longitudinal study of Plasmodium ovale and Plasmodium malariae prevalence and morbidity in a West African population. PLoS ONE. 2014;9:e87169.
Popkin-Hall ZR, Seth MD, Madebe RA, Budodo R, Bakari C, Francis F, et al. Malaria species positivity rates among symptomatic individuals across regions of differing transmission intensities in Mainland Tanzania. J Infect Dis. 2023. https://doi.org/10.1093/infdis/jiad522.
Rogier E, Battle N, Bakari C, Seth MD, Nace D, Herman C, et al. Plasmodium falciparum pfhrp2 and pfhrp3 gene deletions among patients enrolled at 100 health facilities throughout Tanzania: February to July 2021. medRxiv. 2023;223:S81.
Emerson C, Ulimboka S, Lemwayi R, Kinyina A, Nhiga SL, Aaron S, et al. Women attending antenatal care as a sentinel surveillance population for malaria in Geita region, Tanzania: feasibility and acceptability to women and providers. Malar J. 2023;22:1–10.
Gutman JR, Mwesigwa JN, Arnett K, Kangale C, Aaron S, Babarinde D, et al. Using antenatal care as a platform for malaria surveillance data collection: study protocol. Malar J. 2023;22:1–10.
Massicotte P, South A. rnaturalearth: world map data from natural earth. 2023. https://cran.r-project.org/web/packages/rnaturalearth/index.html. Accessed 7 Aug 2023
Abdulraheem MA, Ernest M, Ugwuanyi I, Abkallo HM, Nishikawa S, Adeleke M, et al. High prevalence of Plasmodium malariae and Plasmodium ovale in co-infections with Plasmodium falciparum in asymptomatic malaria parasite carriers in southwestern Nigeria. Int J Parasitol. 2022;52:23–33.
Andolina C, Rek JC, Briggs J, Okoth J, Musiime A, Ramjith J, et al. Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis. 2021;21:1568–78.
Walldorf JA, Cohee LM, Coalson JE, Bauleni A, Nkanaunena K, Kapito-Tembo A, et al. School-age children are a reservoir of malaria infection in Malawi. PLoS ONE. 2015;10:e0134061.
Liu Q, Yan W, Qin C, Du M, Liu M, Liu J. Millions of excess cases and thousands of excess deaths of malaria occurred globally in 2020 during the COVID-19 pandemic. J Glob Health. 2022;12:05045.
Acknowledgements
The authors wish to thank participants and parents/guardians of all children who took part in the surveillance. We acknowledge the contribution of the following project staff and other colleagues who participated in data collection and/or laboratory processing of samples: Raymond Kitengeso, Ezekiel Malecela, Muhidin Kassim, Athanas Mhina, August Nyaki, Juma Tupa, Anangisye Malabeja, Emmanuel Kessy, George Gesase, Tumaini Kamna, Grace Kanyankole, Oswald Osca, Richard Makono, Ildephonce Mathias, Godbless Msaki, Rashid Mtumba, Gasper Lugela, Gineson Nkya, Daniel Chale, Richard Malisa, Sawaya Msangi, Ally Idrisa, Francis Chambo, Kusa Mchaina, Neema Barua, Christian Msokame, Rogers Msangi, Salome Simba, Hatibu Athumani, Mwanaidi Mtui, Rehema Mtibusa, Jumaa Akida, Ambele Yatinga, and Tilaus Gustav. We also acknowledge the finance, administrative and logistic support team at NIMR: Christopher Masaka, Millen Meena, Beatrice Mwampeta, Gracia Sanga, Neema Manumbu, Halfan Mwanga, Arison Ekoni, Twalipo Mponzi, Pendael Nasary, Denis Byakuzana, Alfred Sezary, Emmanuel Mnzava, John Samwel, Daud Mjema, Seth Nguhu, Thomas Semdoe, Sadiki Yusuph, Alex Mwakibinga, Rodrick Ulomi and Andrea Kimboi. We are also grateful to the management of the National Institute for Medical Research, National Malaria Control Program and President's Office-Regional Administration and Local Government (regional administrative secretaries of the four regions, district officials and Community Health Workers from the four regions). Technical and logistics support from the Bill and Melinda Gates Foundation team is highly appreciated. The following reagents were obtained through BEI Resources, NIAID, NIH: Diagnostic Plasmid Containing the Small Subunit Ribosomal RNA Gene (18S) from Plasmodium falciparum, MRA-177; Plasmodium vivax, MRA-178; Plasmodium malariae, MRA-179; and Plasmodium ovale, MRA-180, contributed by Peter A. Zimmerman. Permission to publish the manuscript was sought and obtained from the Director General of NIMR.
Funding
This work was supported, in part, by the Bill & Melinda Gates Foundation [grant number 002202]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. Data collection in Geita was funded by USAID/PMI through Jhpiego and CDC. JJJ also received funding from NIH grant K24AI134990.
Author information
Authors and Affiliations
Contributions
ZRPH, JAB, JJJ and DSI conceived the study. ZRPH performed computational and epidemiological analyses and wrote the manuscript. MDS, RAM, RB, CB and DP collected samples, extracted DNA and performed qPCR analysis. CIM, JAB, JJJ and DSI oversaw the project. FF and DJG contributed to data analysis. JRG and DSI oversaw data collection in Geita and assisted with the statistical analysis. DM, SA, AL and SL contributed data from NMCP and facilitated data collection. JAB, JJJ, JRG, MDS and DSI edited the manuscript. All authors read and approved the final manuscript.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Molecular Surveillance of Malaria in Tanzania (MSMT) study protocol was approved by the Tanzanian Medical Research Coordinating Committee of the National Institute for Medical Research and involved approved standard procedures for informed consent and sample deidentification [13].
Consent for publication
Not applicable.
Competing interests
We declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Regional distribution of samples in random subset and full dataset. Table S2. Age group distribution of samples in random subset and full dataset. Table S3. Sex distribution of samples in random subset and full dataset. Table S4. Species positivity by region and age group.
Additional file 2: Figure S1.
Samples included in analysis by age group and region.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Popkin-Hall, Z.R., Seth, M.D., Madebe, R.A. et al. Prevalence of non-falciparum malaria infections among asymptomatic individuals in four regions of Mainland Tanzania. Parasites Vectors 17, 153 (2024). https://doi.org/10.1186/s13071-024-06242-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06242-4