- Research
- Open access
- Published:
Human Babesia odocoilei and Bartonella spp. co-infections in the Americas
Parasites & Vectors volume 17, Article number: 302 (2024)
Abstract
Background
In recent years, Babesia and Bartonella species co-infections in patients with chronic, nonspecific illnesses have continued to challenge and change the collective medical understanding of “individual pathogen” vector-borne infectious disease dynamics, pathogenesis and epidemiology. The objective of this case series is to provide additional molecular documentation of Babesia odocoilei infection in humans in the Americas and to emphasize the potential for co-infection with a Bartonella species.
Methods
The development of improved and more sensitive molecular diagnostic techniques, as confirmatory methods to assess active infection, has provided increasing clarity to the healthcare community.
Results
Using a combination of different molecular diagnostic approaches, infection with Babesia odocoilei was confirmed in seven people suffering chronic non-specific symptoms, of whom six were co-infected with one or more Bartonella species.
Conclusions
We conclude that infection with Babesia odocoilei is more frequent than previously documented and can occur in association with co-infection with Bartonella spp.
Graphical Abstract
Background
Human babesiosis, an emerging zoonosis caused by apicomplexan protozoa of the genus Babesia, has been described on nearly every continent [1, 2]. Worldwide to date, at least nine different species have been reported to cause infections in human beings: Babesia bigemina, B. crassa (and B. crassa-like), B. divergens, B. duncani, B. microti, B. motasi, B. odocoilei and B. venatorum [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. In the USA, the main Babesia species that infect humans are B. microti, B. duncani and B. divergens-like [2, 48,49,50]. Transmission occurs mainly by tick bite, but there are reports of transmission by transfusion of contaminated blood, organ transplantation and transplacental transmission [51,52,53,54]. In addition to asymptomatic infection, babesiosis can be associated with non-specific symptoms or severe, life-threatening hemolytic anemia, the severity of which can be related to immunosuppressive factors, such as splenectomy [55].
In Canada and the US, Babesia odocoilei, a protozoal species mostly associated with infection in cervids (white tailed deer, elk, reindeer and moose) and musk ox, has also been reported to infect people [47]. A closely related species (in many cases reported as B. odocoilei) has also been reported in red deer from Austria, the Czech Republic, England and Germany [56, 57]. Ixodes scapularis and Dermacentor variabilis are considered the primary tick vectors for transmission of B. odocoilei in the USA and Canada [58,59,60,61,62,63,64,65,66], whereas I. ricinus and I. canisuga have been associated with transmission of B. odocoilei-like organisms in Europe [56, 57].
In 2003, Herwaldt and colleagues [47] first reported the possibility of B. odocoilei infection in two human patients (one from Italy and one from Austria), both experiencing night sweats, chills, fevers, profound fatigue, increased thirst, muscle aches and sleep disturbances, symptoms historically associated with babesiosis. Both patients were seroreactive to B. divergens and seronegative to B. microti antigens. Babesia DNA sequences from both patients were identical and were phylogenetic related to B. odocoilei, a parasite of white-tailed deer in North America. In retrospect, due to limitations associated with species discrimination using the 18S rRNA gene, these individuals were likely infected with B. divergens rather than B. odocoilei, as these two closely related ruminant Babesia form a sister phylogenetic clade [47]. In 2021, Scott and colleagues [39] reported B. odocoilei infection in two humans, both experiencing night sweats, chills, fevers, profound fatigue, increased thirst, muscle aches and sleep disturbances. The 18S rRNA DNA sequences from these two individuals were most similar to B. odocoilei sequences obtained from ticks and cervids in Canada.
Based primarily on serological test results, most often associated with B. duncani as the test antigen, babesiosis has often been diagnosed as a co-infection in patients with Lyme disease, caused by Borrelia burgdorferi, a spirochete also transmitted by I. scapularis. The primary vector for transmission of B. duncani is Ixodes pacificus, a tick species localized to the west coast of North America. To the authors’ knowledge B. duncani DNA has never been amplified from a tick, pet dog (frequently tested by PCR diagnostically), human or wild animal east of the Rocky Mountains. Thus, a discrepancy has existed between human serology and vector epidemiology results, which may have been associated with serological cross-reactivity between B. duncani and B. odocoilei, as reported by Scott and colleagues [39].
Despite clinical and epidemiological support for vector transmission, whether or the extent to which Bartonella spp., and in particular Bartonella henselae, are transmitted by ticks in North America remains undetermined [67,68,69,70,71,72]. Although the common “cat flea” Ctenocephalides felis is the most important vector for B. henselae transmission worldwide, other vectors including woodlouse hunter spiders, rat mites and ants (Australia and the US) have been implicated as a source of B. henselae vector transmission to humans [70, 73,74,75]. In addition to needle stick transmission to a veterinarian, B. henselae DNA has been amplified from dolphins, Beluga whales, and sea otters in the marine environment and mongoose in the Caribbean islands [8, 9, 76,77,78,79,80,81]. Although vectors are the primary modes for B. henselae transmission, viability of this bacteria within aquatic, marine and terrestrial environments may represent an underestimated source for human infections. Currently, the medical importance of the genus Bartonella remains underappreciated and incompletely studied [38]. An important area of emerging research focuses on the potential role of B. henselae as a cause or cofactor in patients with psychoses, schizophrenia and other neuropsychiatric presentations, which makes defining mode(s) of transmission, duration of infection and the medical consequences of chronic infection of the utmost importance [13, 82,83,84,85,86,87,88].
The advent of more sensitive molecular diagnostic techniques continues to change the collective medical understanding of vector-borne infectious disease dynamics, pathogenesis and epidemiology, with important but incompletely understood implications for patients. Droplet digital PCR assays (ddPCRs) were developed and validated in our laboratory to enhance the sensitivity of detection of Babesia, Bartonella and Borrelia spp. DNA in animal and human patient specimens [89, 90]. The enhanced sensitivity of ddPCR facilitated the detection of B. odocoilei DNA in the seven research participants, six of whom were co-infected with one or more Bartonella spp. Additional molecular validation allowed for confirmation of B. odocoilei infection, an emerging human pathogen. The objective of this case series is to provide additional molecular documentation of B. odocoilei infection in humans in the Americas and to emphasize the potential for co-infection with a Bartonella species.
Methods
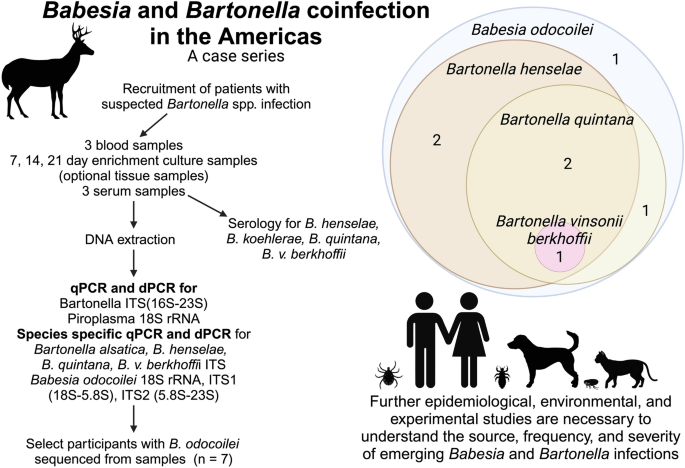
With the cooperation of their attending physician, blood and serum were submitted by all participants. Tissues (two individuals) and an intravenous port sample (another individual) were submitted by three participants. All samples were processed in the Intracellular Pathogens Research Laboratory (IPRL), College of Veterinary Medicine, North Carolina State University, for attempted isolation or molecular detection of a Bartonella species. All study participants provided three blood and serum specimens collected within a 7-day period. These individuals were tested because of a history of arthropod or animal contact as a component of an Institutional Review Board (IRB) approved study entitled "Detection of Bartonella Species in the Blood of People with Extensive Animal Contact" (North Carolina State University Institutional Review Board, IRB#s 4925-03 and 164-08-05). Permission to test for other infectious agents was individually granted. A standardized questionnaire including age, gender, animal and arthropod exposure, outdoor activity, travel, clinical symptoms, duration of illness and comorbid conditions was completed by each individual or by the parents of the two children. The duration of illness varied substantially among individuals, as did prior diagnostic evaluations and previous treatments.
As described previously [87, 91, 92], each participant was tested using five indirect fluorescent antibody (IFA) assays, each representing a unique Bartonella species or genotype. Bartonella vinsonii subsp. berkhoffii (genotypes I and II), B. henselae (strain San Antonio 2), B. koehlerae and B. quintana IgG antibodies were determined using DH82 cell culture-grown bacteria as antigens and following standard IFA techniques with fluorescein conjugated goat anti-human IgG. A sample was considered Bartonella sp. seroreactive if an IFA titer of ≥ 1:64 was obtained for any one or more antigen.
The culture and molecular testing approach used in this study is depicted in Fig. 1 [93]. Following DNA extraction from blood, serum, tissues and an intravenous port sample, amplification of the human hydroxymethylbilane synthase gene was used as the housekeeping human reference gene. The Bartonella spp. intergenic spacer 16S-23S rRNA (ITS) region and Babesia 18S rRNA gene were targeted by quantitative PCR (qPCR, CFXOpus thermocycler, Bio-Rad, Hercules, CA), ddPCR (QX One Droplet Digital PCR, Bio-Rad, Hercules, CA) and dPCR (QIAcuity 5 plex digital PCR, QIAgen Qiagen, Valencia, CA) using primers and probes as previously described [89, 90, 94]. Blood, serum and enrichment blood cultures incubated in Brugge liquid culture media for 7, 14 and 21 days were tested by qPCR and ddPCR targeting the Bartonella 16S-23 S intergenic spacer (ITS) region and the Babesia 18S rRNA gene [89, 90]. DNA was manually extracted (Qiagen DNeasy Blood and Tissue Kit, Qiagen, Valencia, CA, USA) following the manufacturer’s tissue extraction protocol from omental carcinomatosis, uterine wall, fibroid tissues and the intravenous port sample for qPCR and ddPCR testing. A sample was considered PCR positive if qPCR or ddPCR generated a positive result [90]. Babesia 18S rRNA qPCR ddPCR-positive DNA extractions were subsequently tested using two B. odocoilei-specific ITS probes that were developed to confirm the identity of the Babesia species. Sequences were aligned and compared with GenBank sequences using AlignX software (Vector NTI Suite 6.0, InforMax, Inc.).
Results
Demographic data for the seven individuals infected with B. odocoilei are summarized in Table 1. Ages ranged from 2.5 to 62 years old. Six individuals were female. Four individuals were veterinary workers, two were students, one of whom was a veterinarian’s daughter, and one was a pre-school-age child. They resided in four USA states and Mexico. Reported illness duration at the time of testing ranged from days to 14 years. Symptoms reported by individuals on the study questionnaire are listed in Table 2. Fatigue was the most frequently reported symptom, followed by memory loss, headache, irritability/rage/aggression and poor appetite. Night sweats and air hunger, symptoms historically associated with babesiosis, were not respondent options on the questionnaire. Nearly all seven study participants reported exposure to the same arthropod and insect vectors, including fleas, ticks, biting flies, mosquitoes and spiders. No study participant reported exposure to bedbugs, and only participant 4, co-infected with Bartonella henselae and B. quintana, reported louse exposure, although her mother (participant 3) was also co-infected with these same two Bartonella spp. Based upon the medical history, questionnaire responses, prolonged duration of illness or failure to observe a tick for study participant 7, it was not possible to determine the mode or timing of pathogen transmission.
All seven participants were initially determined to be 18S rRNA piroplasma dPCR positive. Infection was confirmed to be a Babesia species based upon DNA sequencing using qPCR amplified DNA. Subsequent DNA sequence comparisons, based on amplification of the ITS1 and/or ITS2 regions, were generated to confirm infection with B. odocoilei. Table 3 provides B. odocoilei 18S rRNA, ITS1 and ITS2 region sequence similarities for blood, enrichment blood culture, tissues and a surgically removed intravenous port for the seven participants compared to B. odocoilei sequences from elk, white-tailed-deer, caribou and reindeer deposited in GenBank.
Six of seven individuals were seroreactive to one or more Bartonella spp. antigens (Table 4). Bartonella henselae DNA was amplified and sequenced from three individuals, whereas infection was detected by B. henselae species-specific probes in two additional participants (Table 4). Bartonella quintana DNA was amplified and sequenced from one individual, whereas infection was detected in three additional participants by B. quintana species-specific probes. Participant 2 was co-infected with B. henselae, B. vinsonii subsp. berkhoffii and B. quintana. A veterinarian residing in northern Michigan was seronegative to four of five Bartonella spp. antigens, and Bartonella DNA was not amplified from her blood, serum, enrichment blood cultures or tissues. The 2.5-year-old child was Bartonella spp. seronegative at acute illness onset; however, the child seroconverted to all five Bartonella spp. antigens after administration of antibiotics (Table 4).
An abbreviated summary of each participants medical history follows:
Participant 1 was diagnosed with peritoneal carcinomatosis 3 months prior to study entry. She had been diagnosed with bartonellosis 5 years earlier and was treated with various oral antibiotic combinations between 2017 and 2022. Despite treatment interventions, she reported progressive fatigue, memory loss, headaches and joint/muscle pain spanning 6 years in duration. Co-infection with B. odocoilei (ITS1 and ITS2 primers) and B. henselae (ITS region) were confirmed by PCR amplification and DNA sequencing from blood or enrichment blood cultures. Neither Bartonella nor Babesia DNA was amplified from omental carcinomatosis tissue obtained 3 weeks after study entry.
Participant 2 developed neuropsychiatric symptoms consistent with Pediatric Acute Onset Neuropsychiatric Syndrome. Until 4 years old, this boy was an extremely verbal, high-functioning child, with normal physical, psychological and academic development. Acutely, he experienced a rapid regression in speech, involving both receptive and expressive language. He also developed acute anxiety, severe deterioration in sleep patterns and additional changes in behavior and personality. According to his father, “the boy’s condition deteriorated to the point where he was almost entirely non-verbal, physically weak, and impaired, and consumed by anxiety that significantly impeded his well-being.” He was treated with clonazepam and clonidine for several years. At the time of study entry, “undiagnosed brain disease of 5 years and 10 months duration” was listed as a working diagnosis, despite having been examined by 12 physicians in various specialties. Babesia odocoilei DNA was amplified using 18S rRNA, ITS1 and ITS2 primers from one of three blood samples, whereas B. henselae, B. quintana and B. vinsonii subsp. berkhoffii were detected in three, two and one samples, respectively, using Bartonella dPCR speciation probes.
Participant 3 reported a history of severe depression spanning many years. She had been evaluated by psychiatrists in Mexico, including the National Institute of Psychiatrists TAC, RM (Instituto Nacional de Psiquiatría Ramon de la Fuente Muñiz in Mexico City, Calzada Mexico-Xochimilco 101, Tlalpan). She had been refractory to a spectrum of psychiatric drugs, participated in a pharmacogenetic study, received neurostimulator therapy and received two cycles of intravenous ketamine. She had previously declined electroconvulsive therapy. As a companion animal veterinarian, she never recalled being bitten by a tick but had experienced flea bites and cat scratches. Other than travel to the Southeastern US for professional meetings, she had never traveled outside of Mexico. At the time of initial research testing, she was medically disabled and reported progressively worsening symptoms. She was co-infected with B. henselae, B. quintana and B. odocoilei. She was treated for bartonellosis with rifampicin and doxycycline for 2 months, after which doxycycline, 100 mg twice daily, was continued for 8 months, during which time she reported symptom resolution and improved memory and was able to stop psychiatric medications. Within months of stopping antibiotics, symptoms, including depression, anxiety, suicidal ideation and memory loss, returned. Bartonella quintana and B. henselae DNA was amplified and sequenced from her blood post-antibiotic treatment. As B. odocoilei infection was only retrospectively confirmed by DNA amplification of the 18S rRNA, ITS1 and ITS2 in 2024 in blood samples obtained 5 months apart, she had not been treated for babesiosis.
Participant 4 (daughter of participant 3) reported a combination of symptoms suggestive of autonomic nervous system dysfunction and neuropsychiatric illness, including suicidal/homicidal thoughts. At 9 years of age, she was diagnosed with oppositional defiant disorder (ODD) because of anxiety, severe depression, headaches, nightmares and hallucinations. She subsequently attempted suicide and over the ensuing years developed tachycardia, repeated urinary infections, homicidal thoughts and cutaneous stretch marks. She had assisted her mother, a veterinarian, in her clinic caring for animal patients for at least 2 years prior to illness onset. On the questionnaire, she reported cat and dog bites in the same year in which she was diagnosed with ODD. Except for a visit to the southwestern US, she had never traveled outside of Mexico. When her January 2022 samples were retrospectively tested, only B. odocoilei DNA was amplified and sequenced (ITS1 and ITS2 regions); however, her September 2022 samples contained both B. odocoilei (as determined by DNA sequences) and B. quintana and B. henselae (determined using species-specific probes) DNA. The mother and daughter were both infected with B. odocoilei, B. quintana and B. henselae.
Participant 5 first entered our IRB-approved study in 2011 at 42 years of age; she reported a 24- year duration of illness. As a veterinary technician, she sought research testing due to a 3-month history of medically refractory migraines, including non-responsiveness to three consecutive daily injections of intravenous dihydroergotamine. Occupationally, and personally, she had frequent contact with animals and arthropod vectors. She had an extensive travel history within the US and had visited Central America and South Africa. She reported bites and scratches from numerous animal species. She was seroreactive to B. henselae San Antonio 2 (SA2) strain type (1:256) but not seroreactive to B. henselae Houston 1, (IFA titer < 1:16) or the four other Bartonella spp. or genotypes. Bartonella henselae SA2 strain DNA was amplified and sequenced from two blood samples collected 4 days apart. When treated for bartonellosis, her migraines resolved. Within 3 months, she seroreverted (B. henselae SA2 IFA titer < 1:16) and was qPCR negative from blood and BAPGM enrichment blood culture DNA extractions. Due to persistence of other symptoms listed in Table 2, she was retested on multiple occasions. Bartonella henselae DNA was amplified from her blood in 2013, 2016 and 2017. Despite treatment with multiple oral and intravenous antibiotics for prolonged durations for bartonellosis, her health continued to deteriorate to the point that she became medically disabled. Between 2019 and 2020, Bartonella spp. ddPCR was positive in blood or in a 14- or 21-day enrichment blood culture. Again, in January 2022, B. henselae DNA was amplified and sequenced from her blood sample. One week later her catheter port was removed. Babesia odocoilei DNA was independently amplified and sequenced from the port and from the catheter, presumably containing biofilm.
Participant 6, a very physically active veterinarian, reported mild, progressive fatigue and insomnia of 3-year duration prior to research testing. Due to endometriosis and uterine fibroids, a hysterectomy was performed 2 weeks after blood and serum were submitted for Bartonella testing. She was only seroreactive to B. quintana. Bartonella spp. DNA was not amplified from blood, serum or enrichment blood cultures. Babesia odocoilei DNA was amplified and sequenced from her uterus (the highest B. odocoilei probe result for any study participant) and from one fibroid.
Participant 7 developed a “bulls eye” rash (Fig. 2) in June 2022 after playing that day in her parents' backyard in Oklahoma. A local pediatrician reported to the parents that she had seen several other children in the city with similar rashes around that period. No tick was attached or observed. Over the ensuing months, the child developed night sweats, knee pain, nightmares and sleep apnea. Despite prior antibiotic treatment, in November 2023, her father reported the following: “She has experienced an array of moderate symptoms over the last few years. She has significant night sweats, frequently complains of knee pain, nightmares, and appears to have sleep apnea at times during the night. There are other symptoms but those are the most common. I'd consider her healthy now, but I have seen some minor nightmares and one night of night sweats in the last few weeks.” Research testing results generated for blood specimens collected between June 2022 and January 2024 are summarized in Table 5. Both she and her father (2024, data not shown) were infected with B. quintana and B. odocoilei with identical ITS1 DNA sequences. Although other participants were piroplasm 18S rRNA dPCR + at multiple testing time points, chronic B. odocoilei infection was confirmed based on DNA sequencing of the 18S rRNA, ITS1 and ITS2 regions in this girl and participant 3.
Photographs taken by the parents of a rapid onset, homogeneous, non-pruritic red rash on the upper left arm (arrows) in a 2.5-year-old girl (study participant 7) co-infected with Babesia odocoilei, Bartonella henselae and Bartonella quintana. A Image obtained at 9:30 p.m., June 6, 2022, when the rash was first noted. B Image obtained at 2 p.m., June 7, 2022, roughly 15 h after the rash was first visualized, illustrating an expansive lesion and bullseye-like appearance. Written permission was granted for publication of the photographs
The datasets generated and analyzed during the current study are available in GenBank and can be accessed through accession numbers PP550637–PP550643; PP592351, PP592352 and PP550653–PP550661; and PP550644–PP550652 for 18S rRNA gene, ITS-1 and ITS-2, respectively. A phylogenetic tree using ITS1 and ITS2 region sequences, compared with closely related species and genera, are depicted in Fig. 3a and b, respectively.
Phylogenetic tree of Babesia ITS1 (A) and ITS2 (B) sequences using HKY + G evolutionary model. The sequences were aligned with other homologous sequences of each gene retrieved from the database (GenBank) using the MAFFT software [95] and edited via Bioedit v. 7.0.5.3 [96]. W-IQ-Tree software was used for choosing the evolutionary model following AIC criterion as well as for phylogenetic analysis inferred the Maximum Likelihood method (available online: http://iqtree.cibiv.univie.ac.at/) [97]. Clade support indices were evaluated through bootstrap analyses of 1000 repetitions. The phylogenetic trees were edited using Treegraph 2.0.56–381 beta software [98]
Discussion
For over 3 decades, our research has primarily focused on the genus Bartonella. The seven individuals in this study were initially tested for Bartonella sp. infection, which was documented in all but one person. Because co-infection with Babesia, Bartonella and Borrelia species has been increasingly reported or suspected in patients with chronic, therapeutically recalcitrant medical symptoms, we developed and validated a multiplex ddPCR assay to amplify DNA of all three genera [89]. Previously, co-infections with these three genera have usually been diagnosed on the basis of serology [99,100,101,102,103]. Indirect diagnostic methods such as serology can only provide evidence for pathogen exposure, can lack specificity due to potential cross-reactivity with other microorganisms or other species within the same genus and can lack sensitivity in immunocompromised patients or when infection is accompanied by a state of immunological anergy [104,105,106]. Due to low level bacteremia and the relapsing nature of Bartonella spp. infections, standard diagnostic methods, such as blood smear examination and PCR (conventional or quantitative/real time) using DNA extracted from blood, have relatively low direct detection sensitivities. Due to these limitations, we used an enrichment culture approach in conjunction with obtaining three blood collections within a 7-day period to enhance the direct detection sensitivity of qPCR and ddPCR for a molecular diagnosis of bartonellosis. Based upon the preliminary results derived from these seven individuals, similar diagnostic sensitivity challenges are to be anticipated when attempting to achieve molecular confirmation of B. odocoilei infections in patient blood or serum specimens. It is important to emphasize that three blood and serum specimens were collected from study participants within a 7-day period and that blood, serum and three enrichment blood cultures incubated in Brugge liquid culture media for 7, 14 and 21 days were tested by qPCR and ddPCR targeting the Bartonella ITS region. Thus, 15 independent DNA extractions from blood serum and enrichment cultures were required to generate the Bartonella spp. PCR results reported in this study. For each participant, most DNA extractions were PCR negative. Also in this study, qPCR lacked sensitivity, as 16 samples (55.2%, 16/29) were piroplasm 18S rRNA gene dPCR positive, whereas all were qPCR negative. Only two (6.9%) samples were piroplasm 18S rRNA qPCR positive and dPCR negative. Similarly, despite extracting DNA from blood, serum and 7-, 14- and 21-day enrichment triple-draw blood cultures (in total 15 independent DNA extractions/participant), only one to three samples were Babesia dPCR positive per participant, and in some instances only 1–2 dots were amplified, reflecting an extremely low B. odocoilei parasitemia. If enrichment culture had not been employed in the testing strategy, B. odocoilei parasitemia would not have been confirmed in participants 1, 4 and 7. Collectively, these results emphasize the inherent challenges in documenting infection with both blood-borne pathogens in patient blood specimens.
Babesia odocoilei species-specific 18S rRNA, ITS-1 and ITS-2 qPCR and dPCR assays developed in this study proved to be reasonably concordant. All seven B. odocoilei 18S rRNA dPCR samples were ITS qPCR positive. Specifically, eight of 11 dPCR-positive samples for B. odocoilei ITS1 region were qPCR positive for the same target gene and seven of the 10 positive samples for the B. odocoilei ITS2 region were qPCR positive. As only 13/41 (31.7%) samples were positive for the specific B. odocoilei assays (either as 18S rRNA, ITS1 and/or ITS2), the difference may be due to concurrent co-infection with another Babesia species (unpublished data). It is also possible that the low parasitemia in these samples prevented detection using these B. odocoilei-specific PCR assays. Thus, obtaining definitive molecular confirmation of B. odocoilei infection in a non-reservoir host remains challenging, despite the enhanced sensitivity of dPCR.
Although blood transfusion transmission of Babesia spp. is a public health concern, ticks are considered the primary if not the sole vector for transmission, including B. odocoilei. However, there is substantial evidence of dog-to-dog Babesia gibsoni transmission via fighting. Bites, particularly bites from American Staffordshire (Pitbull) terriers, are a mode of transmission [107]. As five individuals in this study were veterinary workers, including the daughter of a veterinarian with similar animal exposures, and all reported ownership or exposure to dogs, zoonotic transmission could not be ruled out. The child in this study developed a circumscribed bullseye-like rash after playing in the parent’s yard during a particularly rainy summer period in Oklahoma. An insect bite was suspected, as an attached tick was not seen. The skin lesion was distinctly different from mosquito bites that the child had experienced previously. In addition to a tick bite, it is perhaps prudent to consider other modes of B. odocoilei transmission in future studies.
Although it is increasingly clear that Babesia and Bartonella species can induce longstanding blood-borne intraerythrocytic infections in immunocompetent human patients, the extent to which chronic infection contributes to immune system dysfunction, autoimmune phenomena and non-specific symptoms, such as severe fatigue, is substantially less clear. Thus, the symptoms reported by these individuals cannot be solely or partially attributed to these infections. Participants 1 and 5, diagnosed with bartonellosis years earlier, remained B. henselae seroreactive, and B. henselae DNA was amplified from their blood, despite long-term treatment with multiple antibiotic combinations administered following diagnosis. Participant 3 remained infected with B. quintana post-antibiotic treatment. Based upon duration of illness, participants 1–5 were chronically ill, with non-specific symptoms, as reported previously in bartonellosis patients [108,109,110]. In contrast, the child had an acute onset rash followed by symptoms potentially consistent with babesiosis (night sweats) and bartonellosis (knee pain, nightmares). As participant 6 was a veterinarian, failure to document infection with a Bartonella spp. in blood, serum or tissues was unexpected as was the amplification of B. odocoilei DNA from her uterus and one uterine fibroid. Three independent PCR targets (18S rRNA gene, ITS1 and ITS2), performed at different time points, were positive for her uterine tissue, making DNA carryover or laboratory contamination unlikely. Notably, B. odocoilei DNA was not amplified from her blood, serum or enrichment blood cultures (15 independent DNA extractions), suggesting the possibility of Babesia localization to her reproductive tissues. Experimental transplacental transmission of B. microti has been reported using rats as has natural transplacental transmission of Babesia bovis to cattle, B. caballi to horses, B. gibsoni to dogs, B. microti to mice (Peromyscus leucopus) and cases of suspected B. microti transmission to children [3, 4, 111]. Whether or the extent to which B. odocoilei infection may have contributed to her endometriosis or uterine fibroids is unknown. Endometriosis, a disease of unknown causation, does have immunological features that are reported in association with chronic infections [112]. Also, Babesia and Bartonella transplacental transmission from mother to daughter might be a consideration for participants 3 and 4.
In the context of limitations, it was not possible to determine when or by what mode of transmission individuals in this study were infected with B. odocoilei or a Bartonella species. As the molecular detection of both organisms in patient blood specimens is difficult to achieve, considerable time, effort and testing were performed on multiple samples at different times to obtain an adequate quantity of DNA for successful sequencing. Some participants had been treated for babesiosis, bartonellosis or both infections prior to and after our initial 18S rRNA Babesia focused testing began in 2022. Also, we did not anticipate potential growth of B. odocoilei in enrichment culture, which clearly deserves future research consideration.
Conclusions
Considering the relatively short 1-year (2022) study period with subsequent follow-up for some participants, the wide geographic distribution of study participants, the variable and often non-specific symptoms, and the fact that B. odocoilei DNA was present in blood, tissues, biofilm and a port, we conclude that infection with this Babesia sp. is more prevalent than previously suspected. Also, in contrast to acute babesiosis, which is most often associated with an acute hemolytic anemia or thrombocytopenia, these hematological abnormalities were not reported by study participants, potentially further limiting a physician’s decision to test for babesiosis. The findings reported in this study clearly justify additional applied research to define the medical importance of human B. odocoilei and Bartonella spp. co-infections in Mexico, the USA, and potentially elsewhere.
Availability of data and materials
Sequences analyzed during the current study are available in GenBank under the accession numbers: PP550637-PP550643; PP592351, PP592352, and PP550653-PP550661; PP550644-PP550652 for 18S rRNA gene, ITS-1 and ITS-2, respectively.
References
Kumar A, O’Bryan J, Krause PJ. The global emergence of human babesiosis. Pathogens. 2021;10:11.
Karshima SN, Karshima MN, Ahmed MI. Infection rates, species diversity, and distribution of zoonotic Babesia parasites in ticks: a global systematic review and meta-analysis. Parasitol Res. 2022;121:311–34.
Calvopina M, Montesdeoca-Andrade M, Bastidas-Caldes C, Enriquez S, Rodriguez-Hidalgo R, Aguilar-Rodriguez D, et al. Case report: first report on human infection by tick-borne Babesia bigemina in the Amazon region of Ecuador. Front Public Health. 2023;11:1079042.
Espinosa-Munoz DY, Lopez-Lopez L, Rios-Osorio LA, Gutierrez LA. Detection of Babesia and the associated factors in cattle and humans from Magdalena Medio region. Colombia Comp Immunol Microbiol Infect Dis. 2022;90–91:101900.
Doderer-Lang C, Filisetti D, Badin J, Delale C, Clavier V, Brunet J, et al. Babesia crassa-like human infection indicating need for adapted PCR diagnosis of babesiosis. France Emerg Infect Dis. 2022;28:449–52.
Jia N, Zheng YC, Jiang JF, Jiang RR, Jiang BG, Wei R, et al. Human babesiosis caused by a Babesia crassa-like pathogen: a case series. Clin Infect Dis. 2018;67:1110–9.
Strasek-Smrdel K, Korva M, Pal E, Rajter M, Skvarc M, Avsic-Zupanc T. Case of Babesia crassa-like infection, Slovenia, 2014. Emerg Infect Dis. 2020;26:1038–40.
Gonzalez LM, Castro E, Lobo CA, Richart A, Ramiro R, Gonzalez-Camacho F, et al. First report of Babesia divergens infection in an HIV patient. Int J Infect Dis. 2015;33:202–4.
Jahfari S, Hofhuis A, Fonville M, van der Giessen J, van Pelt W, Sprong H. Molecular detection of tick-borne pathogens in humans with tick bites and erythema migrans, in the Netherlands. PLoS Negl Trop Dis. 2016;10:e0005042.
Kukina IV, Guzeeva TM, Zelya OP, Ganushkina LA. Fatal human babesiosis caused by Babesia divergens in an asplenic host. IDCases. 2018;13:e00414.
Martinot M, Zadeh MM, Hansmann Y, Grawey I, Christmann D, Aguillon S, et al. Babesiosis in immunocompetent patients. Europe Emerg Infect Dis. 2011;17:114–6.
Qi C, Zhou D, Liu J, Cheng Z, Zhang L, Wang L, et al. Detection of Babesia divergens using molecular methods in anemic patients in Shandong Province. China Parasitol Res. 2011;109:241–5.
Centeno-Lima S, do Rosario V, Parreira R, Maia AJ, Freudenthal AM, Nijhof AM, et al. A fatal case of human Babesiosis in Portugal: molecular and phylogenetic analysis. Trop Med Int Health. 2003;8:760–4.
Haapasalo K, Suomalainen P, Sukura A, Siikamaki H, Jokiranta TS. Fatal Babesiosis in man, Finland, 2004. Emerg Infect Dis. 2010;16:1116–8.
Wang J, Zhang S, Yang J, Liu J, Zhang D, Li Y, et al. Babesia divergens in human in Gansu province. China Emerg Microbes Infect. 2019;8:959–61.
Herwaldt BL, de Bruyn G, Pieniazek NJ, Homer M, Lofy KH, Slemenda SB, et al. Babesia divergens-like infection, Washington State. Emerg Infect Dis. 2004;10:622–9.
Bloch EM, Herwaldt BL, Leiby DA, Shaieb A, Herron RM, Chervenak M, et al. The third described case of transfusion-transmitted Babesia duncani. Transfusion. 2012;52:1517–22.
Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, et al. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol. 2006;36:779–89.
Arsuaga M, Gonzalez LM, Lobo CA, de la Calle F, Bautista JM, Azcarate IG, et al. First report of Babesia microti-caused babesiosis in Spain. Vector Borne Zoonotic Dis. 2016;16:677–9.
Gilmore RD Jr, Carpio AM, Kosoy MY, Gage KL. Molecular characterization of the sucB gene encoding the immunogenic dihydrolipoamide succinyltransferase protein of Bartonella vinsonii subsp. berkhoffii and Bartonella quintana. Infect Immun. 2003;71:4818–22.
Gabrielli S, Totino V, Macchioni F, Zuniga F, Rojas P, Lara Y, et al. Human Babesiosis, Bolivia, 2013. Emerg Infect Dis. 2016;22:1445–7.
Hildebrandt A, Hunfeld KP, Baier M, Krumbholz A, Sachse S, Lorenzen T, et al. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J Clin Microbiol Infect Dis. 2007;26:595–601.
Holler JG, Roser D, Nielsen HV, Eickhardt S, Chen M, Lester A, et al. A case of human Babesiosis in Denmark. Travel Med Infect Dis. 2013;11:324–8.
Moniuszko-Malinowska A, Swiecicka I, Dunaj J, Zajkowska J, Czupryna P, Zambrowski G, et al. Infection with Babesia microti in humans with non-specific symptoms in North East Poland. Infect Dis (Lond). 2016;48:537–43.
Jablonska J, Zarnowska-Prymek H, Stanczak J, Kozlowska J, Wiercinska-Drapalo A. Symptomatic co-infection with Babesia microti and Borrelia burgdorferi in patient after international exposure; a challenging case in Poland. Ann Agric Environ Med. 2016;23:387–9.
Jain K, Tagliafierro T, Marques A, Sanchez-Vicente S, Gokden A, Fallon B, et al. Development of a capture sequencing assay for enhanced detection and genotyping of tick-borne pathogens. Sci Rep. 2021;11:12384.
Kim HJ, Kim MJ, Shin HI, Ju JW, Lee HI. Imported human Babesiosis in the Republic of Korea, 2019: two case reports. Parasites Hosts Dis. 2023;61:72–7.
Kim JY, Cho SH, Joo HN, Tsuji M, Cho SR, Park IJ, et al. First case of human Babesiosis in Korea: detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine Babesia. J Clin Microbiol. 2007;45:2084–7.
Lim PL, Chavatte JM, Vasoo S, Yang J. Imported human Babesiosis, Singapore, 2018. Emerg Infect Dis. 2020;26:826–8.
Peniche-Lara G, Balmaceda L, Perez-Osorio C, Munoz-Zanzi C. Human Babesiosis, Yucatan State, Mexico, 2015. Emerg Infect Dis. 2018;24:2061–2.
Ramharter M, Walochnik J, Lagler H, Winkler S, Wernsdorfer WH, Stoiser B, et al. Clinical and molecular characterization of a near fatal case of human babesiosis in Austria. J Travel Med. 2010;17:416–8.
Sayama Y, Zamoto-Niikura A, Matsumoto C, Saijo M, Ishihara C, Matsubayashi K, et al. Analysis of antigen-antibody cross-reactivity among lineages and sublineages of Babesia microti parasites using human babesiosis specimens. Transfusion. 2018;58:1234–44.
Senanayake SN, Paparini A, Latimer M, Andriolo K, Dasilva AJ, Wilson H, et al. First report of human Babesiosis in Australia. Med J Aust. 2012;196:350–2.
Stahl P, Poinsignon Y, Pouedras P, Ciubotaru V, Berry L, Emu B, et al. Case report of the patient source of the Babesia microti R1 reference strain and implications for travelers. J Travel Med. 2018;25(1). https://doi.org/10.1093/jtm/tax073.
Welc-Faleciak R, Pawelczyk A, Radkowski M, Pancewicz SA, Zajkowska J, Sinski E. First report of two asymptomatic cases of human infection with Babesia microti (Franca, 1910) in Poland. Ann Agric Environ Med. 2015;22:51–4.
Zhou X, Li SG, Wang JZ, Huang JL, Zhou HJ, Chen JH, et al. Emergence of human Babesiosis along the border of China with Myanmar: detection by PCR and confirmation by sequencing. Emerg Microbes Infect. 2014;3:e55.
Zhou X, Li SG, Chen SB, Wang JZ, Xu B, Zhou HJ, et al. Co-infections with Babesia microti and Plasmodium parasites along the China-Myanmar border. Infect Dis Poverty. 2013;2:24.
Saito-Ito A, Tsuji M, Wei Q, He S, Matsui T, Kohsaki M, et al. Transfusion-acquired, autochthonous human Babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J Clin Microbiol. 2000;38:4511–6.
Scott JD, Sajid MS, Pascoe EL, Foley JE. Detection of Babesia odocoilei in humans with Babesiosis symptoms. Diagnostics (Basel). 2021;11:947.
Huang L, Sun Y, Huo DD, Xu M, Xia LY, Yang N, et al. Successful treatment with doxycycline monotherapy for human infection with Babesia venatorum (Babesiidae, Sporozoa) in China: a case report and proposal for a clinical regimen. Infect Dis Poverty. 2023;12:67.
Jiang JF, Zheng YC, Jiang RR, Li H, Huo QB, Jiang BG, et al. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: a descriptive study. Lancet Infect Dis. 2015;15:196–203.
Sun Y, Li SG, Jiang JF, Wang X, Zhang Y, Wang H, et al. Babesia venatorum infection in child. China Emerging Infect Dis. 2014;20:896–7.
Hong SH, Kim SY, Song BG, Rho JR, Cho CR, Kim CN, et al. Detection and characterization of an emerging type of Babesia sp. similar to Babesia motasi for the first case of human Babesiosis and ticks in Korea. Emerg Microbes Infect. 2019;8:869–78.
Haselbarth K, Tenter AM, Brade V, Krieger G, Hunfeld KP. First case of human Babesiosis in Germany—Clinical presentation and molecular characterisation of the pathogen. Int J Med Microbiol. 2007;297:197–204.
Bonsergent C, de Carne MC, de la Cotte N, Moussel F, Perronne V, Malandrin L. The new human Babesia sp. FR1 is a European member of the Babesia sp. MO1 Clade. Pathogens. 2021;10:1433.
Man SQ, Qiao K, Cui J, Feng M, Fu YF, Cheng XJ. A case of human infection with a novel Babesia species in China. Inf Dis Poverty. 2016;5:28.
Herwaldt BL, Caccio S, Gherlinzoni F, Aspock H, Slemenda SB, Piccaluga P, et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic Babesiosis in Europe. Emerg Inf Dis. 2003;9:942–8.
Bloch EM, Kumar S, Krause PJ. Persistence of Babesia microti infection in humans. Pathogens. 2019;8(3):102. https://doi.org/10.3390/pathogens8030102.
Krause PJ. Human Babesiosis. Int J Parasitol. 2019;49:165–74.
Radcliffe C, Krause PJ, Grant M. Repeat exchange transfusion for treatment of severe Babesiosis. Transfus Apher Sci. 2019;58:638–40.
Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated Babesiosis in the United States: a description of cases. Ann Intern Med. 2011;155:509–19.
Leiby DA, Johnson ST, Won KY, Nace EK, Slemenda SB, Pieniazek NJ, et al. A longitudinal study of Babesia microti infection in seropositive blood donors. Transfusion. 2014;54:2217–25.
Brennan MB, Herwaldt BL, Kazmierczak JJ, Weiss JW, Klein CL, Leith CP, et al. Transmission of Babesia microti parasites by solid organ transplantation. Emerg Infect Dis. 2016;22:1869–76.
Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366:2397–407.
Waked R, Krause PJ. Human Babesiosis. Infect Dis Clin North Am. 2022;36:655–70.
Gandy S, Medlock J, Cull B, Smith R, Gibney Z, Sewgobind S, et al. Detection of Babesia species in questing Ixodes ricinus ticks in England and Wales. Ticks Tick Borne Dis. 2024;15:102291.
Gray A, Capewell P, Zadoks R, Taggart MA, French AS, Katzer F, et al. Wild deer in the United Kingdom are a potential reservoir for the livestock parasite Babesia divergens. Curr Res Parasitol Vector Borne Dis. 2021;1:100019.
Livengood J, Hutchinson ML, Thirumalapura N, Tewari D. Detection of Babesia, Borrelia, Anaplasma, and Rickettsia spp. in Adult Black-Legged Ticks (Ixodes scapularis) from Pennsylvania, United States, with a Luminex multiplex bead assay. Vector Borne Zoonotic Dis. 2020;20:406–11.
Milnes EL, Thornton G, Leveille AN, Delnatte P, Barta JR, Smith DA, et al. Babesia odocoilei and zoonotic pathogens identified from Ixodes scapularis ticks in southern Ontario. Canada Ticks Tick Borne Dis. 2019;10:670–6.
Scott JD, Clark KL, Durden LA. Presence of Babesia odocoilei and Borrelia burgdorferi sensu stricto in a tick and dual parasitism of Amblyomma inornatum and Ixodes scapularis on a bird in Canada. Healthcare (Basel). 2019;7(1):46. https://doi.org/10.3390/healthcare7010046.
Scott JD, Pascoe EL, Sajid MS, Foley JE. Detection of Babesia odocoilei in Ixodes scapularis ticks collected from songbirds in Ontario and Quebec, Canada. Pathogens. 2020;9:781.
Scott JD, Pascoe EL, Sajid MS, Foley JE. Detection of Babesia odocoilei in Ixodes scapularis ticks collected in Southern Ontario, Canada. Pathogens. 2021;10:327.
Scott JD, Pesapane RR. Detection of Anaplasma phagocytophilum, Babesia odocoilei, Babesia sp., Borrelia burgdorferi sensu lato, and Hepatozoon canis in Ixodes scapularis ticks collected in Eastern Canada. Pathogens. 2021;10:1265.
Steiner FE, Pinger RR, Vann CN, Abley MJ, Sullivan B, Grindle N, et al. Detection of Anaplasma phagocytophilum and Babesia odocoilei DNA in Ixodes scapularis (Acari: Ixodidae) collected in Indiana. J Med Entomol. 2006;43:437–42.
Waldrup KA, Kocan AA, Barker RW, Wagner GG. Transmission of Babesia odocoilei in white-tailed deer (Odocoileus virginianus) by Ixodes scapularis (Acari: Ixodidae). J Wildl Dis. 1990;26:390–1.
Zembsch TE, Bron GM, Paskewitz SM. Evidence for vertical transmission of Babesia odocoilei (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae). J Med Entomol. 2021;58:2484–7.
Reis C, Cote M, Le Rhun D, Lecuelle B, Levin ML, Vayssier-Taussat M, et al. Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlesii. PLoS Negl Trop Dis. 2011;5:e1186.
Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol. 2008;22:1–15.
Cotte V, Bonnet S, Le Rhun D, Le Naour E, Chauvin A, Boulouis HJ, et al. Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis. 2008;14:1074–80.
Mosbacher ME, Klotz S, Klotz J, Pinnas JL. Bartonella henselae and the potential for arthropod vector-borne transmission. Vector Borne Zoonotic Dis. 2011;11:471–7.
Telford SR, Wormser GP. Bartonella spp. transmission by ticks not established. Emerg Infect Dis. 2010;16:379–84.
Vasil’eva IS. Bartonellosis and a possible role of Ixodes ticks (family Ixodidae, order Parasitiformes) in the transmission of pathogenic Bartonella bacteria. Med Parazitol (Mosk). 2005;2:44–8.
Mascarelli PE, Maggi RG, Hopkins S, Mozayeni BR, Trull CL, Bradley JM, et al. Bartonella henselae infection in a family experiencing neurological and neurocognitive abnormalities after woodlouse hunter spider bites. Parasit Vectors. 2013;6:98.
Bradley JM, Mascarelli PE, Trull CL, Maggi RG, Breitschwerdt EB. Bartonella henselae infections in an owner and two Papillon dogs exposed to tropical rat mites (Ornithonyssus bacoti). Vector Borne Zoonotic Dis. 2014;14:703–9.
Halos L, Jamal T, Maillard R, Girard B, Guillot J, Chomel B, et al. Role of Hippoboscidae flies as potential vectors of Bartonella spp. infecting wild and domestic ruminants. Appl Environ Microbiol. 2004;70:6302–5.
Carrasco SE, Chomel BB, Gill VA, Kasten RW, Maggi RG, Breitschwerdt EB, et al. Novel Bartonella infection in northern and southern sea otters (Enhydra lutris kenyoni and Enhydra lutris nereis). Vet Microbiol. 2014;170:325–34.
Harms C, Maggi RG, Breitschwerdt EB, Clemons-Chevis CL, Solangi M, Rotstein DS, et al. Bartonella species detection in captive, stranded and free-ranging cetaceans. Vet Res. 2008;39:59.
Maggi RG, Harms CA, Hohn AA, Pabst DA, McLellan WA, Walton WJ, et al. Bartonella henselae in porpoise blood. Emerg Infect Dis. 2005;11:1894–8.
Maggi RG, Raverty SA, Lester SJ, Huff DG, Haulena M, Ford SL, et al. Bartonella henselae in captive and hunter-harvested beluga (Delphinapterus leucas). J Wildl Dis. 2008;44:871–7.
Valentine KH, Harms CA, Cadenas MB, Birkenheuer AJ, Marr HS, Braun-McNeill J, et al. Bartonella DNA in loggerhead sea turtles. Emerg Infect Dis. 2007;13:949–50.
Oliveira AM, Maggi RG, Woods CW, Breitschwerdt EB. Suspected needle stick transmission of Bartonella vinsonii subspecies berkhoffii to a veterinarian. J Vet Intern Med. 2010;24:1229–32.
Canneti B, Cabo-Lopez I, Puy-Nunez A, Garcia Garcia JC, Cores FJ, Trigo M, et al. Neurological presentations of Bartonella henselae infection. Neurol Sci. 2019;40:261–8.
Kaufman DL, Kogelnik AM, Mozayeni RB, Cherry NA, Breitschwerdt EB. Neurological and immunological dysfunction in two patients with Bartonella henselae bacteremia. Clin Case Rep. 2017;5:931–5.
Balakrishnan N, Ericson M, Maggi R, Breitschwerdt EB. Vasculitis, cerebral infarction and persistent Bartonella henselae infection in a child. Parasit Vectors. 2016;9:254.
Carpi G, Walter KS, Mamoun CB, Krause PJ, Kitchen A, Lepore TJ, et al. Babesia microti from humans and ticks hold a genomic signature of strong population structure in the United States. BMC Genomics. 2016;17:888.
Breitschwerdt EB, Maggi RG, Lantos PM, Woods CW, Hegarty BC, Bradley JM. Bartonella vinsonii subsp. berkhoffii and Bartonella henselae bacteremia in a father and daughter with neurological disease. Parasit Vectors. 2010;3:29.
Breitschwerdt EB, Maggi RG, Nicholson WL, Cherry NA, Woods CW. Bartonella sp. bacteremia in patients with neurological and neurocognitive dysfunction. J Clin Microbiol. 2008;46:2856–61.
Baylor P, Garoufi A, Karpathios T, Lutz J, Mogelof J, Moseley D. Transverse myelitis in 2 patients with Bartonella henselae infection (cat scratch disease). Clin Infect Dis. 2007;45:e42–5.
Maggi R, Breitschwerdt EB, Qurollo B, Miller JC. Development of a multiplex droplet digital PCR assay for the detection of Babesia, Bartonella, and Borrelia species. Pathogens. 2021;10:1462.
Maggi RG, Richardson T, Breitschwerdt EB, Miller JC. Development and validation of a droplet digital PCR assay for the detection and quantification of Bartonella species within human clinical samples. J Microbiol Methods. 2020;176:106022.
Lantos PM, Maggi RG, Ferguson B, Varkey J, Park LP, Breitschwerdt EB, et al. Detection of Bartonella species in the blood of veterinarians and veterinary technicians: a newly recognized occupational hazard? Vector Borne Zoonotic Dis. 2014;14:563–70.
Breitschwerdt EB, Maggi RG. Bartonella quintana and Bartonella vinsonii subsp. vinsonii bloodstream co-infection in a girl from North Carolina, USA. Med Microbiol Immunol. 2019;208:101–7.
Pultorak EL, Maggi RG, Mascarelli PE, Breitschwerdt EB. Serial testing from a 3-day collection period by use of the Bartonella Alphaproteobacteria growth medium platform may enhance the sensitivity of Bartonella species detection in bacteremic human patients. J Clin Microbiol. 2013;51:1673–7.
Lashnits E, Maggi R, Jarskog F, Bradley J, Breitschwerdt E, Frohlich F. Schizophrenia and Bartonella spp. infection: a pilot case-control study. Vector Borne Zoonotic Dis. 2021;21:413–21.
Katoh S, Honda S, Watanabe T, Suzuki S, Ishino M, Kitahara T, et al. Atrial endothelial impairment through Toll-like receptor 4 signaling causes atrial thrombogenesis. Heart Vessels. 2014;29(2):263–72. https://doi.org/10.1007/s00380-013-0369-3.
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999;41:95–8.
Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44(W1):W232–5. https://doi.org/10.1093/nar/gkw256.
Stover BC, Muller KF. TreeGraph 2: combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics. 2010;11:7. https://doi.org/10.1186/1471-2105-11-7.
Johnson L, Shapiro M, Mankoff J. Removing the mask of average treatment effects in chronic Lyme disease research using big data and subgroup analysis. Healthcare (Basel). 2018;6(4):124. https://doi.org/10.3390/healthcare6040124.
Stricker RB, Johnson L. Lyme disease: the promise of Big Data, companion diagnostics and precision medicine. Infect Drug Resist. 2016;9:215–9.
Stricker RB, Johnson L. Lyme disease: the next decade. Infect Drug Resist. 2011;4:1–9.
Mayne PJ. Emerging incidence of Lyme Borreliosis, Babesiosis, bartonellosis, and granulocytic ehrlichiosis in Australia. Int J Gen Med. 2011;4:845–52.
Horowitz RI, Freeman PR. Precision medicine: the role of the MSIDS model in defining, diagnosing, and treating chronic Lyme disease/post treatment Lyme disease syndrome and other chronic illness: Part 2. Healthcare (Basel). 2018;6(4):129. https://doi.org/10.3390/healthcare6040129.
Maggi RG, Birkenheuer AJ, Hegarty BC, Bradley JM, Levy MG, Breitschwerdt EB. Comparison of serological and molecular panels for diagnosis of vector-borne diseases in dogs. Parasit Vectors. 2014;7:127.
Maggi RG, Breitschwerdt EB. Potential limitations of the 16S–23S rRNA intergenic region for molecular detection of Bartonella species. J Clin Microbiol. 2005;43:1171–6.
Maggi RG, Kramer F. A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasit Vectors. 2019;12:145.
Birkenheuer AJ, Levy MG, Savary KC, Gager RB, Breitschwerdt EB. Babesia gibsoni infections in dogs from North Carolina. J Am Anim Hosp Assoc. 1999;35:125–8.
Oteo JA, Maggi R, Portillo A, Bradley J, Garcia-Alvarez L, San-Martin M, et al. Prevalence of Bartonella spp. by culture, PCR and serology, in veterinary personnel from Spain. Parasit Vectors. 2017;10:553.
Breitschwerdt EB, Maggi RG, Quach C, Bradley JM. Bartonella spp. bloodstream infection in a Canadian family. Vector Borne Zoonotic Dis. 2019;19:234–41.
Portillo A, Maggi R, Oteo JA, Bradley J, Garcia-Alvarez L, San-Martin M, et al. Bartonella spp. prevalence (serology, culture, and PCR) in sanitary workers in La Rioja Spain. Pathogens. 2020;9(3):189. https://doi.org/10.3390/pathogens9030189.
Beattie JF, Michelson ML, Holman PJ. Acute babesiosis caused by Babesia divergens in a resident of Kentucky. N Engl J Med. 2002;347:697–8.
Maksym RB, Hoffmann-Mlodzianowska M, Skibinska M, Rabijewski M, Mackiewicz A, Kieda C. Immunology and immunotherapy of endometriosis. J Clin Microbiol. 2021;10(24):5879. https://doi.org/10.3390/jcm10245879.
Liedig C, Neupane P, Lashnits E, Breitschwerdt EB, Maggi RG. Blood supplementation enhances Bartonella henselae growth and molecular detection of bacterial DNA in liquid culture. Microbiol Spectr. 2023;11:e0512622.
Acknowledgements
The authors thank the study participants and parents for participating in or contributing to this study and Chance Liedig and Lily Bartone for facilitating this investigation and assisting in sample acquisition and Bartonella or Babesia serological and molecular testing.
This paper has been sponsored by Elanco Animal Health in the framework of the CVBD® World Forum Symposium.
Funding
This research was supported through donations to the Bartonella/Vector Borne Diseases Research Fund at the North Carolina State University College of Veterinary Medicine, through a grant from the Steven & Alexandra Cohen Foundation, and by the state of North Carolina. The funding agencies were not involved in the design or any aspect of the study.
Author information
Authors and Affiliations
Contributions
RM and EBB conceived the study. RM, ACC and EBB contributed to the conception and design of the study. RM, ACC and COM designed the study protocol. RM, ACC and EK carried out specimen collections and sample analysis. RM, ACC and EK carried out the analysis and interpretation of data. RM and ACC drafted the manuscript, and RM, ACC, COM and EBB revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Testing of individuals done as a component of an Institutional Review Board (IRB) approved study entitled: Detection of Bartonella Species in the Blood of People with Extensive Animal Contact (North Carolina State University Institutional Review Board, IRB#s 4925-03 and 164-08-05). Permission to test for other infectious agents was individually granted.
Consent for publication
Not applicable.
Competing interests
In conjunction with Dr. S. Sontakke and North Carolina State University, E.B. Breitschwerdt holds US Patent No. 7,115,385 Media and Methods for Cultivation of Microorganisms, which was issued on October 3rd, 2006. He is a co-founder, shareholder and Chief Scientific Officer for Galaxy Diagnostics, a company that provides advanced diagnostic testing for the detection of Bartonella spp. infections. Dr. Ricardo Maggi is a co-founder and the Chief Technical Officer for Galaxy Diagnostics Inc. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Maggi, R.G., Calchi, A.C., Moore, C.O. et al. Human Babesia odocoilei and Bartonella spp. co-infections in the Americas. Parasites Vectors 17, 302 (2024). https://doi.org/10.1186/s13071-024-06385-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06385-4


